Lovastatin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lovastatin is a HMG-CoA Reductase Inhibitor that is FDA approved for the {{{indicationType}}} of prevention of coronary heart disease, hyperlipidemia, limitations of use. Common adverse reactions include abdominal pain, constipation, arthralgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hyperlipidemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia (Fredrickson Types IIa and IIb)
- Dosing Information
- 20-60 mg/day, in single doses taken in the evening at bedtime.
Prevention of Coronary Heart Disease
- Dosing Information
- 20 to 60 mg ORALLY once daily at bedtime; adjust dose at intervals of 4 weeks or more
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Lovastatin in adult patients.
Non–Guideline-Supported Use
Atrial Fibrillation, Prophylaxis
Cerebrovascular Accident, Prophylaxis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lovastatin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Lovastatin in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Lovastatin in pediatric patients.
Contraindications
- Concomitant administration of strong CYP3A inhibitors
- Concomitant administration of erythromycin
- Hypersensitivity to any component of this product
- Women who are pregnant or may become pregnant
- Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol. Additionally, there is no apparent benefit to therapy during pregnancy, and safety in pregnant women has not been established. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and the lack of known clinical benefit with continued use during pregnancy.
- Nursing mothers
- Because another drug in this class passes into breast milk, and because HMG-CoA reductase inhibitors have the potential to cause serious adverse reactions in nursing infants.
Warnings
Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including Altoprev®. These risks can occur at any dose level, but increase in a dose-dependent manner. Predisposing factors for myopathy include advanced age (≥65 years), female gender, renal impairment, and inadequately treated hypothyroidism. In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20-40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily.
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
All patients starting therapy with Altoprev®, or whose dose of Altoprev® is being increased, should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing Altoprev. Altoprev therapy should be discontinued immediately if myopathy is diagnosed or suspected.
Altoprev® therapy should be discontinued if markedly elevated creatine kinase (CK) levels occur or myopathy is diagnosed or suspected. Altoprev® therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; dehydration; major surgery; trauma; severe metabolic, endocrine, and electrolyte disorders; or uncontrolled epilepsy.
Drug Interactions that can cause skeletal muscle effects
- Strong CYP3A Inhibitors
- The risk of myopathy and rhabdomyolysis is increased by high levels of statin activity in plasma. Lovastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs which inhibit this metabolic pathway can raise the plasma levels of lovastatin and may increase the risk of myopathy. Co-administration of these drugs with Altoprev is contraindicated. If treatment with strong CYP3A inhibitors is unavoidable, therapy with Altoprev should be suspended during the course of treatment.
- Erythromycin
- Co-administration of erythromycin with Altoprev is contraindicated. If treatment with erythromycin is unavoidable, therapy with Altoprev should be suspended during the course of treatment.
- Gemfibrozil
- Avoid the combined use of Altoprev with gemfibrozil.
- Other lipid-lowering drugs (other fibrates, or lipid-lowering doses (≥ 1 g/day) of niacin
- Use caution when prescribing other fibrates or lipid-lowering doses (≥ 1 g/day) of niacin with Altoprev, as these agents can cause myopathy when given alone and the risk is increased when they are coadministered with Altoprev. Carefully weigh the expected benefit of further alterations in lipid levels by the combined use of lovastatin with other fibrates or niacin against the potential risks of these combinations.
- Cyclosporine
- Avoid the combined use of Altoprev with cyclosporine.
- Danazol, diltiazem, dronedarone or verapamil with higher doses of lovastatin
- Do not exceed 20 mg of Altoprev daily in patients receiving concomitant therapy with danazol, diltiazem, dronedarone or verapamil. Weigh carefully the benefits of the use of Altoprev in patients receiving danazol, diltiazem, dronedarone or verapamil against the risks of these combinations.
- Amiodarone
- Do not exceed 40 mg of Altoprev daily in patients receiving concomitant therapy with amiodarone. Avoid the combined use of Altoprev at doses exceeding 40 mg daily with amiodarone unless the clinical benefit is likely to outweigh the increased risk of myopathy. The concomitant use of higher doses of a closely related member of the HMG-CoA reductase inhibitor class with amiodarone increased the risk of myopathy/rhabdomyolysis.
- Colchicine
- There have been cases of myopathy, including rhabdomyolysis, reported in patients receiving lovastatin coadministered with colchicine. Use caution when prescribing lovastatin with colchicine.
- Ranolazine
- Concomitant use of ranolazine and Altoprev may increase the risk of myopathy, including rhabdomyolysis. Consider dose adjustment of Altoprev if coadministering with ranolazine.
Prescribing recommendations for interacting agents are summarized in the table below.

Liver Enzyme Abnormalities
Increases in serum transaminases (aspartate aminotransferase [AST] or alanine aminotransferase [ALT]) have been reported with HMG-CoA reductase inhibitors, including Altoprev®.
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials [see Adverse Reactions (6.1)]. When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels.
It is recommended that liver enzyme tests be obtained prior to initiating therapy with Altoprev and repeated as clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including lovastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with Altoprev, promptly interrupt therapy. If an alternate etiology is not found, do not restart Altoprev. The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Active liver disease or unexplained transaminase elevations are contraindications to the use of Altoprev®.
Altoprev®
In controlled clinical trials (467 patients treated with Altoprev® and 329 patients treated with lovastatin immediate-release) no meaningful differences in transaminase elevations between the two treatments were observed.
Lovastatin
In the EXCEL study [see Clinical Studies (14)], the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin immediate-release, symptomatic liver disease has been reported rarely at all dosages.
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (>3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin immediate-release and placebo groups [18 (0.6%) vs. 11 (0.3%)]. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin immediate-release group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including lovastatin.
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and as such might theoretically blunt adrenal and/or gonadal steroid production. Results of clinical trials with drugs in this class have been inconsistent with regard to drug effects on basal and reserve steroid levels. However, clinical studies have shown that lovastatin does not reduce basal plasma cortisol concentration or impair adrenal reserve, and does not reduce basal plasma testosterone concentration. Another HMG-CoA reductase inhibitor has been shown to reduce the plasma testosterone response to HCG. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied in adequate numbers of male patients. The effects, if any, on the pituitary-gonadal axis in premenopausal women are unknown. Patients treated with lovastatin who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if an HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., spironolactone, cimetidine) that may decrease the levels or activity of endogenous steroid hormones.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials with lovastatin, (467 patients with mean exposure to study drug of approximately 11.6 weeks), 3.2% of patients were discontinued due to adverse reactions. This was similar to the discontinuation rate in the placebo (2/34, 5.9%) and lovastatin immediate-release (3.3%) treatment groups. Pooled results from clinical trials with v show that the most frequently reported adverse reactions in the lovastatin group were infection, headache and accidental injury. Similar incidences of these adverse reactions were seen in the lovastatin and placebo groups. In controlled clinical trials, clinical adverse reactions reported in >5% of patients in any treatment group are shown inthe table below.

AFCAPS/TexCAPS
In AFCAPS/TexCAPS involving 6,605 participants treated with 20-40 mg/day of lovastatin immediate-release (n=3,304) or placebo (n=3,301), the safety and tolerability profile of the group treated with lovastatin immediate-release was comparable to that of the group treated with placebo during a median of 5.1 years of follow-up.
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (>3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin immediate-release and placebo groups [18 (0.6%) vs. 11 (0.3%)]. The starting dose of lovastatin immediate-release was 20 mg/day; 50% of the lovastatin immediate-release treated participants were titrated to 40 mg/day at Week 18. Of the 18 participants on lovastatin immediate-release with consecutive elevations of either ALT or AST, 11 (0.7%) elevations occurred in participants taking 20 mg/day, while 7 (0.4%) elevations occurred in participants titrated to 40 mg/day. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin immediate-release group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Altoprev® and/or are class effects of HMG CoA reductase inhibitors (statins). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Skeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias.
- There have been rare reports of immune-mediated necrotizing myopathy associated with statin use.
- Neurological: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, dizziness, vertigo, paresthesia, peripheral neuropathy, peripheral nerve palsy, psychic disturbances, anxiety, insomnia, depression.
- There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
- Hypersensitivity Reactions: An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
- Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver; and rarely, cirrhosis, fulminant hepatic necrosis, and hepatoma; anorexia, vomiting, fatal and non-fatal hepatic failure.
- Skin: alopecia, pruritus. A variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails) have been reported.
- Reproductive: gynecomastia, loss of libido, erectile dysfunction.
- Eye: progression of cataracts (lens opacities), ophthalmoplegia.
- Laboratory Abnormalities: elevated transaminases, alkaline phosphatase, γ-glutamyl transpeptidase, and bilirubin; thyroid function abnormalities.
Drug Interactions
Drug interaction studies have not been performed with Altoprev®. The types, frequencies and magnitude of drug interactions that may be encountered when Altoprev® is administered with other drugs may differ from the drug interactions encountered with the lovastatin immediate-release formulation. In addition, as the drug exposure with Altoprev® 60 mg is greater than that with lovastatin immediate-release 80 mg (maximum recommended dose), the severity and magnitude of drug interactions that may be encountered with Altoprev® 60 mg are not known. It is therefore recommended that the following precautions and recommendations for the concomitant administration of lovastatin immediate-release with other drugs be interpreted with caution, and that the monitoring of the pharmacologic effects of Altoprev® and/or other concomitantly administered drugs be undertaken where appropriate.
- Strong CYP 3A Inhibitors
- Lovastatin is metabolized by CYP3A4 but has no CYP3A inhibitory activity; therefore it is not expected to affect the plasma concentrations of other drugs metabolized by CYP3A. Strong inhibitors of CYP3A (e.g., itraconazole, ketoconazole, posaconazole, voriconazole, clarithromycin, telithromycin, HIV protease inhibitors, boceprevir, telaprevir, and nefazodone), increase the risk of myopathy by reducing the elimination of lovastatin. The use of lovastatin with strong CYP3A inhibitors is contraindicated.
- Erythromycin
- Do not use Altoprev concomitantly with erythromycin.
- Interactions With Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
- The risk of myopathy is also increased by the following lipid-lowering drugs that are not strong CYP3A inhibitors, but which can cause myopathy when given alone.
- Gemfibrozil – Avoid the concomitant use of Altoprev with gemfibrozil.
- Other fibrates - Use caution when prescribing Altoprev with other fibrates.
- Niacin (nicotinic acid) (≥1 g/day)
- Use caution when prescribing Altoprev with lipid-modifying (≥1 g/day) doses of niacin.
- Cyclosporine
- Avoid the concomitant use of Altoprev with cyclosporine.
- Danazol, Diltiazem, Dronedarone or Verapamil
- Do not exceed 20 mg of Altoprev daily in patients receiving concomitant therapy with danazol, diltiazem, dronedarone, or verapamil.
- Amiodarone
- Do not exceed 40 mg of Altoprev daily in patients receiving concomitant therapy with amiodarone.
- Coumarin Anticoagulants
- In a small clinical trial in which lovastatin was administered to warfarin treated patients, no effect on prothrombin time was detected. However, another HMG-CoA reductase inhibitor has been found to produce a less than two second increase in prothrombin time in healthy volunteers receiving low doses of warfarin. Also, bleeding and/or increased prothrombin time has been reported in a few patients taking coumarin anticoagulants concomitantly with lovastatin. In patients taking anticoagulants, prothrombin time should be determined before starting Altoprev and frequently enough during early therapy to ensure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of Altoprev is changed, the same procedure should be repeated. Lovastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
- Colchicine
- Cases of myopathy, including rhabdomyolysis have been reported with lovastatin coadministered with colchicine. Exercise caution when prescribing Altoprev with colchicine.
- Ranolazine
- The risk of myopathy, including rhabdomyolysis, may be increased by concomitant administration of ranolazine. Exercise caution when prescribing Altoprev with ranolazine. Dose adjustment of Altoprev may be necessary during coadministration with ranolazine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X Safety in pregnant women has not been established. Lovastatin immediate-release has been shown to produce skeletal malformations at plasma levels 40 times the human exposure (for mouse fetus) and 80 times the human exposure (for rat fetus) based on mg/m2 surface area (doses were 800 mg/kg/day). No drug-induced changes were seen in either species at multiples of 8 times (rat) or 4 times (mouse) based on surface area. No evidence of malformations was noted in rabbits at exposures up to 3 times the human exposure (dose of 15 mg/kg/day, highest tolerated dose of lovastatin immediate-release).
Rare reports of congenital anomalies have been received following intrauterine exposure to HMG-CoA reductase inhibitors. In a review2 of approximately 100 prospectively followed pregnancies in women exposed to lovastatin immediate-release or another structurally related HMG-CoA reductase inhibitor, the incidences of congenital anomalies, spontaneous abortions and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate only to exclude a 3 to 4-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. As safety in pregnant women has not been established and there is no apparent benefit to therapy with Altoprev® during pregnancy [see Contraindications (4)], treatment should be immediately discontinued as soon as pregnancy is recognized. Altoprev® should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazard.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lovastatin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lovastatin during labor and delivery.
Nursing Mothers
It is not known whether lovastatin is excreted in human milk. Because a small amount of another drug in this class is excreted in human breast milk and because of the potential for serious adverse reactions in nursing infants, women taking Altoprev® should not nurse their infants.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Because pediatric patients are not likely to benefit from cholesterol lowering for at least a decade and because experience with this drug is limited (no studies in subjects below the age of 20 years), treatment of pediatric patients with Altoprev® is not recommended at this time.
Geriatic Use
Of the 467 patients who received Altoprev® in controlled clinical studies, 18% were 65 years and older. Of the 297 patients who received Altoprev® in uncontrolled clinical studies, 22% were 65 years and older. No overall differences in effectiveness or safety were observed between these patients and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Thus, lower starting doses of Altoprev® are recommended for elderly patients.
In pharmacokinetic studies with lovastatin immediate-release, the mean plasma level of HMG-CoA reductase inhibitory activity was shown to be approximately 45% higher in elderly patients between 70-78 years of age compared with patients between 18-30 years of age; however, clinical study experience in the elderly indicates that dosage adjustment based on this age-related pharmacokinetic difference is not needed. In the two large clinical studies conducted with lovastatin immediate-release (EXCEL and AFCAPS/TexCAPS), 21% (3094/14850) of patients were ≥65 years of age. Lipid-lowering efficacy with lovastatin was at least as great in elderly patients compared with younger patients, and there were no overall differences in safety over the 20 to 80 mg dosage range.
Gender
There is no FDA guidance on the use of Lovastatin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lovastatin with respect to specific racial populations.
Renal Impairment
In a study of patients with severe renal impairment (creatinine clearance 10–30 mL/min), the plasma concentrations of total inhibitors after a single dose of lovastatin were approximately two-fold higher than those in healthy volunteers.
Hepatic Impairment
There is no FDA guidance on the use of Lovastatin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lovastatin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lovastatin in patients who are immunocompromised.
Others
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
- After oral administration of lovastatin immediate-release to mice the median lethal dose observed was >15 g/m2.
- Five healthy human volunteers have received up to 200 mg of lovastatin as a single dose without clinically significant adverse experiences. A few cases of accidental overdosage with lovastatin immediate-release have been reported; no patients had any specific symptoms, and all patients recovered without sequelae. The maximum dose taken was 5 g to 6 g.
- Until further experience is obtained, no specific treatment of overdosage with lovastatin can be recommended.
- The dialyzability of lovastatin and its metabolites in man is not known at present.
Pharmacology
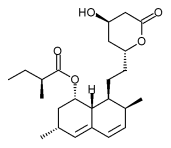
| |
Lovastatin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Lovastatin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Lovastatin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lovastatin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Lovastatin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lovastatin Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.