Liposarcoma pathophysiology: Difference between revisions
Joao Silva (talk | contribs) |
Ahmed Younes (talk | contribs) (→Images) |
||
| (56 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Liposarcoma}} | {{Liposarcoma}} | ||
{{CMG}} {{AE}} {{JS}} | {{CMG}} {{AE}} {{JS}}{{Sab}} | ||
==Overview== | ==Overview== | ||
Liposarcoma is the most common [[sarcoma]] of [[soft tissue]]. The [[pathogenesis]] of liposarcoma depends on the [[Histology|histological]] sub-type. The two sub-types, well differentiated and dedifferentiated, are the two most commonly occurring liposarcomas. The majority of well differentiated liposarcomas arise in the [[retroperitoneum]]. The [[chromosome]] region 12q13-15, is rich in [[protooncogene]]s, including the ''[[CHOP]]'', ''[[Cyclin-dependent kinase 4|CDK4]]'', ''[[MDM2]]'', ''HMGI-C'', ''GLI'', ''SAS'', ''OS1'', and the ''[[OS9 (gene)|OS9]]'', all of which play an important role in the [[pathogenesis]] of many [[neoplasms]]. Liposarcoma is associated with [[Genetic disorder|genetic conditions]] like [[Li-Fraumeni syndrome]]. Liposarcoma usually presents as a mass which often resembles [[lipoma]] and is [[Nodular|multinodular]]. [[Microscopic]] [[pathological]] findings of liposarcoma depend on the sub-type. | |||
== | ==Pathophysiology== | ||
=== Pathogenesis === | |||
=== | *Liposarcoma is the most common [[sarcoma]] of [[soft tissue]].<ref>{{Cite journal | ||
| author = [[Kate Lynn J. Bill]], [[Lucia Casadei]], [[Bethany C. Prudner]], [[Hans Iwenofu]], [[Anne M. Strohecker]] & [[Raphael E. Pollock]] | |||
= | | title = Liposarcoma: molecular targets and therapeutic implications | ||
| journal = [[Cellular and molecular life sciences : CMLS]] | |||
| volume = 73 | |||
| issue = 19 | |||
| pages = 3711–3718 | |||
| year = 2016 | |||
| month = October | |||
| doi = 10.1007/s00018-016-2266-2 | |||
| pmid = 27173057 | |||
}}</ref> | |||
*The two sub-types, well differentiated and dedifferentiated, are the two most commonly occurring liposarcomas.<ref>{{Cite journal | |||
| author = [[Kate Lynn J. Bill]], [[Lucia Casadei]], [[Bethany C. Prudner]], [[Hans Iwenofu]], [[Anne M. Strohecker]] & [[Raphael E. Pollock]] | |||
| title = Liposarcoma: molecular targets and therapeutic implications | |||
| journal = [[Cellular and molecular life sciences : CMLS]] | |||
| volume = 73 | |||
| issue = 19 | |||
| pages = 3711–3718 | |||
| year = 2016 | |||
| month = October | |||
| doi = 10.1007/s00018-016-2266-2 | |||
| pmid = 27173057 | |||
}}</ref> | |||
*The majority of well differentiated liposarcomas arise in the [[retroperitoneum]].<ref>{{Cite journal | |||
| author = [[N. Azumi]], [[J. Curtis]], [[R. L. Kempson]] & [[M. R. Hendrickson]] | |||
| title = Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 11 | |||
| issue = 3 | |||
| pages = 161–183 | |||
| year = 1987 | |||
| month = March | |||
| pmid = 3826477 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[Emily Z. Keung]], [[Jason L. Hornick]], [[Monica M. Bertagnolli]], [[Elizabeth H. Baldini]] & [[Chandrajit P. Raut]] | |||
| title = Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery | |||
| journal = [[Journal of the American College of Surgeons]] | |||
| volume = 218 | |||
| issue = 2 | |||
| pages = 206–217 | |||
| year = 2014 | |||
| month = February | |||
| doi = 10.1016/j.jamcollsurg.2013.10.009 | |||
| pmid = 24315890 | |||
}}</ref> | |||
*The [[tumor]] can also arise in the deep [[soft tissue]] of the [[thigh]], [[mediastinum]], and paratesticular area.<ref>{{Cite journal | |||
| author = [[N. Azumi]], [[J. Curtis]], [[R. L. Kempson]] & [[M. R. Hendrickson]] | |||
| title = Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 11 | |||
| issue = 3 | |||
| pages = 161–183 | |||
| year = 1987 | |||
| month = March | |||
| pmid = 3826477 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[Emily Z. Keung]], [[Jason L. Hornick]], [[Monica M. Bertagnolli]], [[Elizabeth H. Baldini]] & [[Chandrajit P. Raut]] | |||
| title = Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery | |||
| journal = [[Journal of the American College of Surgeons]] | |||
| volume = 218 | |||
| issue = 2 | |||
| pages = 206–217 | |||
| year = 2014 | |||
| month = February | |||
| doi = 10.1016/j.jamcollsurg.2013.10.009 | |||
| pmid = 24315890 | |||
}}</ref> | |||
*[[Retroperitoneum|Retroperitoneal]] [[Tumor|tumors]] are more likely to recur.<ref>{{Cite journal | |||
| author = [[N. Azumi]], [[J. Curtis]], [[R. L. Kempson]] & [[M. R. Hendrickson]] | |||
| title = Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 11 | |||
| issue = 3 | |||
| pages = 161–183 | |||
| year = 1987 | |||
| month = March | |||
| pmid = 3826477 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[Elizabeth Fabre-Guillevin]], [[Jean-Michel Coindre]], [[Nicolas de Saint Aubain Somerhausen]], [[Francoise Bonichon]], [[Eberhard Stoeckle]] & [[Nguyen Binh Bui]] | |||
| title = Retroperitoneal liposarcomas: follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas | |||
| journal = [[Cancer]] | |||
| volume = 106 | |||
| issue = 12 | |||
| pages = 2725–2733 | |||
| year = 2006 | |||
| month = June | |||
| doi = 10.1002/cncr.21933 | |||
| pmid = 16688768 | |||
}}</ref> | |||
*The dedifferentiated sub-type arises as a primary or [[de novo]] [[lesion]] in majority of the cases.<ref name="GhadimiAl-Zaid2011">{{cite journal|last1=Ghadimi|first1=Markus P.|last2=Al-Zaid|first2=Tariq|last3=Madewell|first3=John|last4=Peng|first4=Tingsheng|last5=Colombo|first5=Chiara|last6=Hoffman|first6=Aviad|last7=Creighton|first7=Chad J.|last8=Zhang|first8=Yiqun|last9=Zhang|first9=Anna|last10=Lazar|first10=Alexander J.|last11=Pollock|first11=Raphael E.|last12=Lev|first12=Dina|title=Diagnosis, Management, and Outcome of Patients with Dedifferentiated Liposarcoma Systemic Metastasis|journal=Annals of Surgical Oncology|volume=18|issue=13|year=2011|pages=3762–3770|issn=1068-9265|doi=10.1245/s10434-011-1794-0}}</ref><ref>{{Cite journal | |||
| author = [[G. Lahat]], [[D. A. Anaya]], [[X. Wang]], [[D. Tuvin]], [[D. Lev]] & [[R. E. Pollock]] | |||
| title = Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches | |||
| journal = [[Annals of surgical oncology]] | |||
| volume = 15 | |||
| issue = 6 | |||
| pages = 1585–1593 | |||
| year = 2008 | |||
| month = June | |||
| doi = 10.1245/s10434-007-9805-x | |||
| pmid = 18398663 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[H. L. Evans]], [[K. K. Khurana]], [[B. L. Kemp]] & [[A. G. Ayala]] | |||
| title = Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 18 | |||
| issue = 11 | |||
| pages = 1150–1157 | |||
| year = 1994 | |||
| month = November | |||
| pmid = 7943536 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[W. H. Henricks]], [[Y. C. Chu]], [[J. R. Goldblum]] & [[S. W. Weiss]] | |||
| title = Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 21 | |||
| issue = 3 | |||
| pages = 271–281 | |||
| year = 1997 | |||
| month = March | |||
| pmid = 9060596 | |||
}}</ref> | |||
*The dedifferentiated sub-type is clinically more aggressive than the well differentiated liposarcoma.<ref name="SingerAntonescu2003">{{cite journal|last1=Singer|first1=Samuel|last2=Antonescu|first2=Cristina R.|last3=Riedel|first3=Elyn|last4=Brennan|first4=Murray F.|title=Histologic Subtype and Margin of Resection Predict Pattern of Recurrence and Survival for Retroperitoneal Liposarcoma|journal=Transactions of the ... Meeting of the American Surgical Association|volume=121|year=2003|pages=52–65|issn=0066-0833|doi=10.1097/01.sla.0000086542.11899.38}}</ref><ref>{{Cite journal | |||
| author = [[G. Lahat]], [[D. A. Anaya]], [[X. Wang]], [[D. Tuvin]], [[D. Lev]] & [[R. E. Pollock]] | |||
| title = Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches | |||
| journal = [[Annals of surgical oncology]] | |||
| volume = 15 | |||
| issue = 6 | |||
| pages = 1585–1593 | |||
| year = 2008 | |||
| month = June | |||
| doi = 10.1245/s10434-007-9805-x | |||
| pmid = 18398663 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[Emily Z. Keung]], [[Jason L. Hornick]], [[Monica M. Bertagnolli]], [[Elizabeth H. Baldini]] & [[Chandrajit P. Raut]] | |||
| title = Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery | |||
| journal = [[Journal of the American College of Surgeons]] | |||
| volume = 218 | |||
| issue = 2 | |||
| pages = 206–217 | |||
| year = 2014 | |||
| month = February | |||
| doi = 10.1016/j.jamcollsurg.2013.10.009 | |||
| pmid = 24315890 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[Khin Thway]], [[Robin L. Jones]], [[Jonathan Noujaim]], [[Shane Zaidi]], [[Aisha B. Miah]] & [[Cyril Fisher]] | |||
| title = Dedifferentiated Liposarcoma: Updates on Morphology, Genetics, and Therapeutic Strategies | |||
| journal = [[Advances in anatomic pathology]] | |||
| volume = 23 | |||
| issue = 1 | |||
| pages = 30–40 | |||
| year = 2016 | |||
| month = January | |||
| doi = 10.1097/PAP.0000000000000101 | |||
| pmid = 26645460 | |||
}}</ref> | |||
==Genetics== | |||
====Well-Differentiated Liposarcoma==== | |||
* The [[chromosome]] region 12q13-15, is rich in [[protooncogene]]s, including the ''[[CHOP]]'', ''[[Cyclin-dependent kinase 4|CDK4]]'', ''[[MDM2]]'', ''HMGI-C'', ''GLI'', ''SAS'', ''OS1'', and the ''[[OS9 (gene)|OS9]]'', all of which play an important role in the [[pathogenesis]] of many [[neoplasms]]. | |||
* [[Amplification]] of some of these regions, with concomitant [[amplification]] of their [[Protein|proteins]], has clearly been demonstrated in liposarcoma.<ref name="pmid10727978">{{cite journal| author=Dei Tos AP, Doglioni C, Piccinin S, Sciot R, Furlanetto A, Boiocchi M et al.| title=Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. | journal=J Pathol | year= 2000 | volume= 190 | issue= 5 | pages= 531-6 | pmid=10727978 | doi=10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10727978 }} </ref> | |||
* The difference between [[malignant]] and [[benign]] masses resides in the amount of rearranged [[gene]] present in each [[Tissue (biology)|tissue]].<ref name="pmid10727978">{{cite journal| author=Dei Tos AP, Doglioni C, Piccinin S, Sciot R, Furlanetto A, Boiocchi M et al.| title=Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. | journal=J Pathol | year= 2000 | volume= 190 | issue= 5 | pages= 531-6 | pmid=10727978 | doi=10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10727978 }} </ref> | |||
==== | ==Associated Conditions== | ||
* Liposarcoma is associated with [[Genetic disorder|genetic conditions]] like [[Li-Fraumeni syndrome]]. | |||
==Gross Pathology== | |||
*Liposarcoma usually presents as a mass which often resembles [[lipoma]] and is [[Nodular|multinodular]]. | |||
*Cut surface may be soft or firm and is yellow in color. | |||
*Focal mucinous area may be seen. | |||
*Areas of [[necrosis]] may be noted. | |||
==Microscopic Pathology== | |||
Each liposarcoma sub-type has specific characteristics: | |||
===Well-Differentiated Liposarcoma=== | |||
====Sclerosing Liposarcoma==== | |||
* The particular [[histological]] finding in this type of well differentiated liposarcoma is the identification of distinctive [[stromal]] [[Cell (biology)|cells]] distributed across the [[Tissue (biology)|tissue]], which are associated with lipoblasts filled with multiple [[vacuoles]]. | |||
* This association forms a [[collagenous]] background of fibrillary appearance. | |||
* In certain cases, the [[Fiber|fibrous]] component of the [[neoplasm]] may occupy most of its mass.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> | |||
====Adipocytic Liposarcoma==== | |||
* Frequently composed by [[adipocytes]] with different [[Cell (biology)|cell]] sizes, [[Hyperchromicity|hyperchromasia]], and [[Cell nucleus|nuclear]] atypia. | |||
* [[Fibrous]] [[Septum|septa]] may be identified surrounding [[adipocytes]], containing [[Hyperchromicity|hyperchromatic]] [[stromal cells]]. | |||
* Besides these two types of [[Cell (biology)|cells]], mono- or multi-[[Vacuole|vacuolated]] lipoblasts may also be identified. | |||
* Lipoblasts are characterized by the presence of single (mono) or multiple (multi) [[Periphery|peripheral]] [[Cytoplasm|cytoplasmic]] [[Vacuole|vacuoles]] that press on the [[Hyperchromicity|hyperchromatic]] [[Cell nucleus|nucleus]].<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> | |||
* In general, [[Adipocyte|adipocytic]] [[neoplasms]] are often identified by the presence of these lipoblasts. | |||
* Additionally, lipoblasts may infrequently be absent, which makes the [[diagnosis]] of [[Adipocyte|adipocytic]] [[neoplasm]] more difficult but possible with the help of other [[Histology|histological]] features.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> | |||
====Inflammatory Liposarcoma==== | |||
* Its [[Adipocyte|adipocytic]] nature may be misidentified due to the heavy [[Chronic inflammation|chronic inflammatory]] infiltrate. | |||
* The [[Inflammation|inflammatory]] component is frequently composed of different [[Lymphocyte|lympho]]-[[Plasma cell|plasmacytic]] aggregates. These tend to be predominantly formed by a specific type of [[B-cell]], or less commonly [[T-cells]] may populate the [[Inflammation|inflammatory]] aggregate.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref><ref name="pmid9158675">{{cite journal| author=Kraus MD, Guillou L, Fletcher CD| title=Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 5 | pages= 518-27 | pmid=9158675 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9158675 }} </ref><ref name="pmid9255251">{{cite journal| author=Argani P, Facchetti F, Inghirami G, Rosai J| title=Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 8 | pages= 884-95 | pmid=9255251 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9255251 }} </ref> | |||
--> | ==== '''Spindle Cell Lipocarcinoma''' ==== | ||
* This is a rare [[adult]]-type of well-differentiated liposarcoma. | |||
* It results from the [[Cell growth|proliferation]] of [[neural]]-like [[spindle cells]], which are organized in a [[fibrous]] structure, that contains lipoblasts.<ref name="pmid8067512">{{cite journal| author=Dei Tos AP, Mentzel T, Newman PL, Fletcher CD| title=Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases. | journal=Am J Surg Pathol | year= 1994 | volume= 18 | issue= 9 | pages= 913-21 | pmid=8067512 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8067512 }} </ref><ref name="pmid5">{{cite journal| author=Hendrickson WA, Ward KB| title=Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin. | journal=Biochem Biophys Res Commun | year= 1975 | volume= 66 | issue= 4 | pages= 1349-56 | pmid=5 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5 }} </ref> | |||
===Dedifferentiated Liposarcoma=== | ===De-differentiated Liposarcoma=== | ||
* In this form of liposarcoma, there is a transition from a low-grade differentiation to a high-grade differentiation within the same mass of well-differentiated liposarcoma.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref><ref name="pmid534388">{{cite journal| author=Evans HL| title=Liposarcoma: a study of 55 cases with a reassessment of its classification. | journal=Am J Surg Pathol | year= 1979 | volume= 3 | issue= 6 | pages= 507-23 | pmid=534388 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=534388 }} </ref><ref name="pmid192432">{{cite journal| author=Dahlin DC, Unni KK, Matsuno T| title=Malignant (fibrous) histiocytoma of bone--fact or fancy?. | journal=Cancer | year= 1977 | volume= 39 | issue= 4 | pages= 1508-16 | pmid=192432 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=192432 }} </ref> | |||
* 90% of dedifferentiation from well-differentiated liposarcomas occur in [[Primary tumor|primary tumors]], while the remaining 10% occur in recurrent [[Neoplasm|neoplasms]].<ref name="GhadimiAl-Zaid20112">{{cite journal|last1=Ghadimi|first1=Markus P.|last2=Al-Zaid|first2=Tariq|last3=Madewell|first3=John|last4=Peng|first4=Tingsheng|last5=Colombo|first5=Chiara|last6=Hoffman|first6=Aviad|last7=Creighton|first7=Chad J.|last8=Zhang|first8=Yiqun|last9=Zhang|first9=Anna|last10=Lazar|first10=Alexander J.|last11=Pollock|first11=Raphael E.|last12=Lev|first12=Dina|title=Diagnosis, Management, and Outcome of Patients with Dedifferentiated Liposarcoma Systemic Metastasis|journal=Annals of Surgical Oncology|volume=18|issue=13|year=2011|pages=3762–3770|issn=1068-9265|doi=10.1245/s10434-011-1794-0}}</ref><ref>{{Cite journal | |||
| author = [[G. Lahat]], [[D. A. Anaya]], [[X. Wang]], [[D. Tuvin]], [[D. Lev]] & [[R. E. Pollock]] | |||
| title = Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches | |||
| journal = [[Annals of surgical oncology]] | |||
| volume = 15 | |||
| issue = 6 | |||
| pages = 1585–1593 | |||
| year = 2008 | |||
| month = June | |||
| doi = 10.1245/s10434-007-9805-x | |||
| pmid = 18398663 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[H. L. Evans]], [[K. K. Khurana]], [[B. L. Kemp]] & [[A. G. Ayala]] | |||
| title = Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 18 | |||
| issue = 11 | |||
| pages = 1150–1157 | |||
| year = 1994 | |||
| month = November | |||
| pmid = 7943536 | |||
}}</ref><ref>{{Cite journal | |||
| author = [[W. H. Henricks]], [[Y. C. Chu]], [[J. R. Goldblum]] & [[S. W. Weiss]] | |||
| title = Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation | |||
| journal = [[The American journal of surgical pathology]] | |||
| volume = 21 | |||
| issue = 3 | |||
| pages = 271–281 | |||
| year = 1997 | |||
| month = March | |||
| pmid = 9060596 | |||
}}</ref> | |||
===Myxoid Liposarcoma=== | ===Myxoid Liposarcoma=== | ||
* They have a non-[[Homogeneity|homogenous]] appearance with [[Cyst|cystic]] and solid components. | |||
===Round Cell Liposarcoma=== | ===Round Cell Liposarcoma=== | ||
* It is a high-grade liposarcoma which is a poorly differentiated form of myxoid sarcoma. | |||
* It has a very poor [[prognosis]] and often [[Metastasis|metastasizes]] to the [[retroperitoneum]], [[pleural cavity]], [[soft tissue]], or [[pelvis]] and very rarely to the [[Lung|lungs]]. | |||
* [[Microscopic|Microscopically]], it consists of small, round, or [[spindle cells]] with sparse [[eosinophilic]] and [[Granule (cell biology)|granular]] [[cytoplasm]] and large [[Cell nucleus|nuclei]]. | |||
* It may have scattered lipoblasts and areas of [[necrosis]]. | |||
=== | ===Pleiomorphic Liposarcoma=== | ||
* [[Pleomorphism|Pleomorphic]] [[Cell (biology)|cells]] may be identified with enlarged round to bizarre [[Cell nucleus|nuclei]]. | |||
== | ==Images== | ||
{| | |||
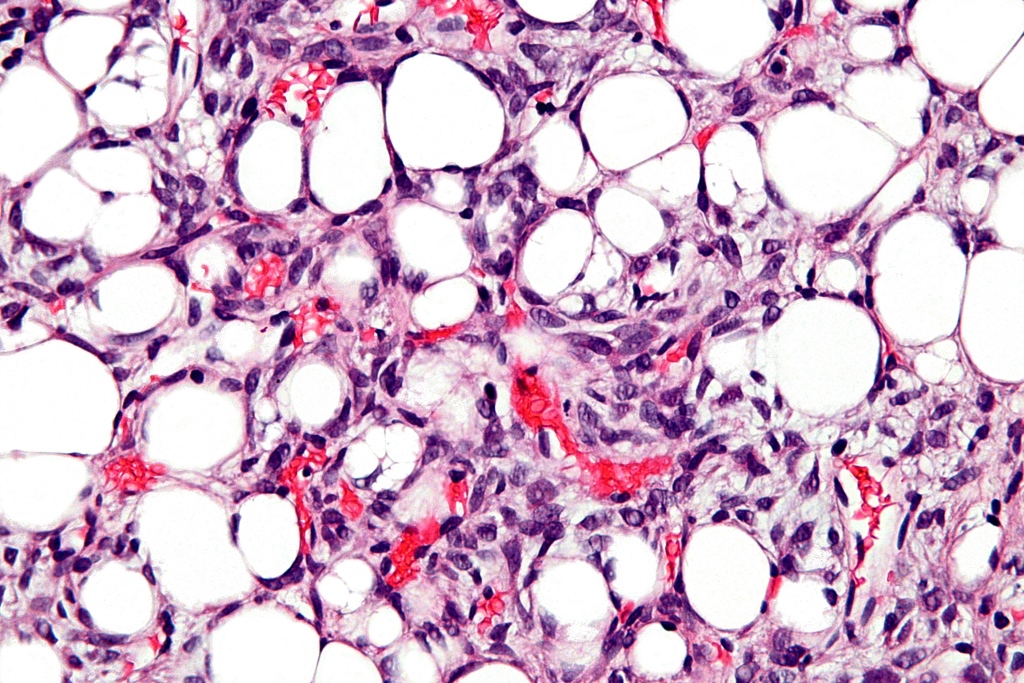
|[[File:Well Differentiated Liposarcoma.jpg|left|thumb|250px|Well Differentiated Liposarcoma. Image courtesy of Wikimedia Commons]] | |||
|[[File:Dedifferentiated Liposarcoma.jpg|center|thumb|250px|Dedifferentiated Liposarcoma. Image courtesy of Wikimedia Commons]] | |||
|[[File:Dedifferentiated Liposarcoma 2.jpg|right|thumb|250px|Dedifferentiated Liposarcoma. Image courtesy of Wikimedia Commons]] | |||
|- | |||
|[[File:Dedifferentiated Liposarcoma 3.jpg|left|thumb|250px|Dedifferentiated Liposarcoma. Image courtesy of Wikimedia Commons]] | |||
|[[File:Dedifferentiated Liposarcoma 4.jpg|center|thumb|250px|Dedifferentiated Liposarcoma. Image courtesy of Wikimedia Commons]] | |||
|} | |||
==References== | ==References== | ||
| Line 54: | Line 268: | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Types of cancer]] | [[Category:Types of cancer]] | ||
[[Category:Surgery]] | [[Category:Surgery]] | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
Latest revision as of 16:48, 27 May 2019
|
Liposarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Liposarcoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Liposarcoma pathophysiology |
|
Risk calculators and risk factors for Liposarcoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]Sabawoon Mirwais, M.B.B.S, M.D.[3]
Overview
Liposarcoma is the most common sarcoma of soft tissue. The pathogenesis of liposarcoma depends on the histological sub-type. The two sub-types, well differentiated and dedifferentiated, are the two most commonly occurring liposarcomas. The majority of well differentiated liposarcomas arise in the retroperitoneum. The chromosome region 12q13-15, is rich in protooncogenes, including the CHOP, CDK4, MDM2, HMGI-C, GLI, SAS, OS1, and the OS9, all of which play an important role in the pathogenesis of many neoplasms. Liposarcoma is associated with genetic conditions like Li-Fraumeni syndrome. Liposarcoma usually presents as a mass which often resembles lipoma and is multinodular. Microscopic pathological findings of liposarcoma depend on the sub-type.
Pathophysiology
Pathogenesis
- Liposarcoma is the most common sarcoma of soft tissue.[1]
- The two sub-types, well differentiated and dedifferentiated, are the two most commonly occurring liposarcomas.[2]
- The majority of well differentiated liposarcomas arise in the retroperitoneum.[3][4]
- The tumor can also arise in the deep soft tissue of the thigh, mediastinum, and paratesticular area.[5][6]
- Retroperitoneal tumors are more likely to recur.[7][8]
- The dedifferentiated sub-type arises as a primary or de novo lesion in majority of the cases.[9][10][11][12]
- The dedifferentiated sub-type is clinically more aggressive than the well differentiated liposarcoma.[13][14][15][16]
Genetics
Well-Differentiated Liposarcoma
- The chromosome region 12q13-15, is rich in protooncogenes, including the CHOP, CDK4, MDM2, HMGI-C, GLI, SAS, OS1, and the OS9, all of which play an important role in the pathogenesis of many neoplasms.
- Amplification of some of these regions, with concomitant amplification of their proteins, has clearly been demonstrated in liposarcoma.[17]
- The difference between malignant and benign masses resides in the amount of rearranged gene present in each tissue.[17]
Associated Conditions
- Liposarcoma is associated with genetic conditions like Li-Fraumeni syndrome.
Gross Pathology
- Liposarcoma usually presents as a mass which often resembles lipoma and is multinodular.
- Cut surface may be soft or firm and is yellow in color.
- Focal mucinous area may be seen.
- Areas of necrosis may be noted.
Microscopic Pathology
Each liposarcoma sub-type has specific characteristics:
Well-Differentiated Liposarcoma
Sclerosing Liposarcoma
- The particular histological finding in this type of well differentiated liposarcoma is the identification of distinctive stromal cells distributed across the tissue, which are associated with lipoblasts filled with multiple vacuoles.
- This association forms a collagenous background of fibrillary appearance.
- In certain cases, the fibrous component of the neoplasm may occupy most of its mass.[18]
Adipocytic Liposarcoma
- Frequently composed by adipocytes with different cell sizes, hyperchromasia, and nuclear atypia.
- Fibrous septa may be identified surrounding adipocytes, containing hyperchromatic stromal cells.
- Besides these two types of cells, mono- or multi-vacuolated lipoblasts may also be identified.
- Lipoblasts are characterized by the presence of single (mono) or multiple (multi) peripheral cytoplasmic vacuoles that press on the hyperchromatic nucleus.[18]
- In general, adipocytic neoplasms are often identified by the presence of these lipoblasts.
- Additionally, lipoblasts may infrequently be absent, which makes the diagnosis of adipocytic neoplasm more difficult but possible with the help of other histological features.[18]
Inflammatory Liposarcoma
- Its adipocytic nature may be misidentified due to the heavy chronic inflammatory infiltrate.
- The inflammatory component is frequently composed of different lympho-plasmacytic aggregates. These tend to be predominantly formed by a specific type of B-cell, or less commonly T-cells may populate the inflammatory aggregate.[18][19][20]
Spindle Cell Lipocarcinoma
- This is a rare adult-type of well-differentiated liposarcoma.
- It results from the proliferation of neural-like spindle cells, which are organized in a fibrous structure, that contains lipoblasts.[21][22]
De-differentiated Liposarcoma
- In this form of liposarcoma, there is a transition from a low-grade differentiation to a high-grade differentiation within the same mass of well-differentiated liposarcoma.[18][23][24]
- 90% of dedifferentiation from well-differentiated liposarcomas occur in primary tumors, while the remaining 10% occur in recurrent neoplasms.[25][26][27][28]
Myxoid Liposarcoma
- They have a non-homogenous appearance with cystic and solid components.
Round Cell Liposarcoma
- It is a high-grade liposarcoma which is a poorly differentiated form of myxoid sarcoma.
- It has a very poor prognosis and often metastasizes to the retroperitoneum, pleural cavity, soft tissue, or pelvis and very rarely to the lungs.
- Microscopically, it consists of small, round, or spindle cells with sparse eosinophilic and granular cytoplasm and large nuclei.
- It may have scattered lipoblasts and areas of necrosis.
Pleiomorphic Liposarcoma
- Pleomorphic cells may be identified with enlarged round to bizarre nuclei.
Images
References
- ↑ Kate Lynn J. Bill, Lucia Casadei, Bethany C. Prudner, Hans Iwenofu, Anne M. Strohecker & Raphael E. Pollock (2016). "Liposarcoma: molecular targets and therapeutic implications". Cellular and molecular life sciences : CMLS. 73 (19): 3711–3718. doi:10.1007/s00018-016-2266-2. PMID 27173057. Unknown parameter
|month=ignored (help) - ↑ Kate Lynn J. Bill, Lucia Casadei, Bethany C. Prudner, Hans Iwenofu, Anne M. Strohecker & Raphael E. Pollock (2016). "Liposarcoma: molecular targets and therapeutic implications". Cellular and molecular life sciences : CMLS. 73 (19): 3711–3718. doi:10.1007/s00018-016-2266-2. PMID 27173057. Unknown parameter
|month=ignored (help) - ↑ N. Azumi, J. Curtis, R. L. Kempson & M. R. Hendrickson (1987). "Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases". The American journal of surgical pathology. 11 (3): 161–183. PMID 3826477. Unknown parameter
|month=ignored (help) - ↑ Emily Z. Keung, Jason L. Hornick, Monica M. Bertagnolli, Elizabeth H. Baldini & Chandrajit P. Raut (2014). "Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery". Journal of the American College of Surgeons. 218 (2): 206–217. doi:10.1016/j.jamcollsurg.2013.10.009. PMID 24315890. Unknown parameter
|month=ignored (help) - ↑ N. Azumi, J. Curtis, R. L. Kempson & M. R. Hendrickson (1987). "Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases". The American journal of surgical pathology. 11 (3): 161–183. PMID 3826477. Unknown parameter
|month=ignored (help) - ↑ Emily Z. Keung, Jason L. Hornick, Monica M. Bertagnolli, Elizabeth H. Baldini & Chandrajit P. Raut (2014). "Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery". Journal of the American College of Surgeons. 218 (2): 206–217. doi:10.1016/j.jamcollsurg.2013.10.009. PMID 24315890. Unknown parameter
|month=ignored (help) - ↑ N. Azumi, J. Curtis, R. L. Kempson & M. R. Hendrickson (1987). "Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases". The American journal of surgical pathology. 11 (3): 161–183. PMID 3826477. Unknown parameter
|month=ignored (help) - ↑ Elizabeth Fabre-Guillevin, Jean-Michel Coindre, Nicolas de Saint Aubain Somerhausen, Francoise Bonichon, Eberhard Stoeckle & Nguyen Binh Bui (2006). "Retroperitoneal liposarcomas: follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas". Cancer. 106 (12): 2725–2733. doi:10.1002/cncr.21933. PMID 16688768. Unknown parameter
|month=ignored (help) - ↑ Ghadimi, Markus P.; Al-Zaid, Tariq; Madewell, John; Peng, Tingsheng; Colombo, Chiara; Hoffman, Aviad; Creighton, Chad J.; Zhang, Yiqun; Zhang, Anna; Lazar, Alexander J.; Pollock, Raphael E.; Lev, Dina (2011). "Diagnosis, Management, and Outcome of Patients with Dedifferentiated Liposarcoma Systemic Metastasis". Annals of Surgical Oncology. 18 (13): 3762–3770. doi:10.1245/s10434-011-1794-0. ISSN 1068-9265.
- ↑ G. Lahat, D. A. Anaya, X. Wang, D. Tuvin, D. Lev & R. E. Pollock (2008). "Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches". Annals of surgical oncology. 15 (6): 1585–1593. doi:10.1245/s10434-007-9805-x. PMID 18398663. Unknown parameter
|month=ignored (help) - ↑ H. L. Evans, K. K. Khurana, B. L. Kemp & A. G. Ayala (1994). "Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma". The American journal of surgical pathology. 18 (11): 1150–1157. PMID 7943536. Unknown parameter
|month=ignored (help) - ↑ W. H. Henricks, Y. C. Chu, J. R. Goldblum & S. W. Weiss (1997). "Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation". The American journal of surgical pathology. 21 (3): 271–281. PMID 9060596. Unknown parameter
|month=ignored (help) - ↑ Singer, Samuel; Antonescu, Cristina R.; Riedel, Elyn; Brennan, Murray F. (2003). "Histologic Subtype and Margin of Resection Predict Pattern of Recurrence and Survival for Retroperitoneal Liposarcoma". Transactions of the ... Meeting of the American Surgical Association. 121: 52–65. doi:10.1097/01.sla.0000086542.11899.38. ISSN 0066-0833.
- ↑ G. Lahat, D. A. Anaya, X. Wang, D. Tuvin, D. Lev & R. E. Pollock (2008). "Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches". Annals of surgical oncology. 15 (6): 1585–1593. doi:10.1245/s10434-007-9805-x. PMID 18398663. Unknown parameter
|month=ignored (help) - ↑ Emily Z. Keung, Jason L. Hornick, Monica M. Bertagnolli, Elizabeth H. Baldini & Chandrajit P. Raut (2014). "Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery". Journal of the American College of Surgeons. 218 (2): 206–217. doi:10.1016/j.jamcollsurg.2013.10.009. PMID 24315890. Unknown parameter
|month=ignored (help) - ↑ Khin Thway, Robin L. Jones, Jonathan Noujaim, Shane Zaidi, Aisha B. Miah & Cyril Fisher (2016). "Dedifferentiated Liposarcoma: Updates on Morphology, Genetics, and Therapeutic Strategies". Advances in anatomic pathology. 23 (1): 30–40. doi:10.1097/PAP.0000000000000101. PMID 26645460. Unknown parameter
|month=ignored (help) - ↑ 17.0 17.1 Dei Tos AP, Doglioni C, Piccinin S, Sciot R, Furlanetto A, Boiocchi M; et al. (2000). "Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours". J Pathol. 190 (5): 531–6. doi:10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. PMID 10727978.
- ↑ 18.0 18.1 18.2 18.3 18.4 Dei Tos AP (2000). "Liposarcoma: new entities and evolving concepts". Ann Diagn Pathol. 4 (4): 252–66. doi:10.1053/adpa.2000.8133. PMID 10982304.
- ↑ Kraus MD, Guillou L, Fletcher CD (1997). "Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma". Am J Surg Pathol. 21 (5): 518–27. PMID 9158675.
- ↑ Argani P, Facchetti F, Inghirami G, Rosai J (1997). "Lymphocyte-rich well-differentiated liposarcoma: report of nine cases". Am J Surg Pathol. 21 (8): 884–95. PMID 9255251.
- ↑ Dei Tos AP, Mentzel T, Newman PL, Fletcher CD (1994). "Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases". Am J Surg Pathol. 18 (9): 913–21. PMID 8067512.
- ↑ Hendrickson WA, Ward KB (1975). "Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin". Biochem Biophys Res Commun. 66 (4): 1349–56. PMID 5.
- ↑ Evans HL (1979). "Liposarcoma: a study of 55 cases with a reassessment of its classification". Am J Surg Pathol. 3 (6): 507–23. PMID 534388.
- ↑ Dahlin DC, Unni KK, Matsuno T (1977). "Malignant (fibrous) histiocytoma of bone--fact or fancy?". Cancer. 39 (4): 1508–16. PMID 192432.
- ↑ Ghadimi, Markus P.; Al-Zaid, Tariq; Madewell, John; Peng, Tingsheng; Colombo, Chiara; Hoffman, Aviad; Creighton, Chad J.; Zhang, Yiqun; Zhang, Anna; Lazar, Alexander J.; Pollock, Raphael E.; Lev, Dina (2011). "Diagnosis, Management, and Outcome of Patients with Dedifferentiated Liposarcoma Systemic Metastasis". Annals of Surgical Oncology. 18 (13): 3762–3770. doi:10.1245/s10434-011-1794-0. ISSN 1068-9265.
- ↑ G. Lahat, D. A. Anaya, X. Wang, D. Tuvin, D. Lev & R. E. Pollock (2008). "Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches". Annals of surgical oncology. 15 (6): 1585–1593. doi:10.1245/s10434-007-9805-x. PMID 18398663. Unknown parameter
|month=ignored (help) - ↑ H. L. Evans, K. K. Khurana, B. L. Kemp & A. G. Ayala (1994). "Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma". The American journal of surgical pathology. 18 (11): 1150–1157. PMID 7943536. Unknown parameter
|month=ignored (help) - ↑ W. H. Henricks, Y. C. Chu, J. R. Goldblum & S. W. Weiss (1997). "Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation". The American journal of surgical pathology. 21 (3): 271–281. PMID 9060596. Unknown parameter
|month=ignored (help)