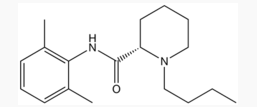
Levobupivacaine
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | Hepatic |
| Elimination half-life | 2–2.6 hours |
| Excretion | Renal 70%, faecal 24% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H28N2O |
| Molar mass | 288.43 g/mol |
|
WikiDoc Resources for Levobupivacaine |
|
Articles |
|---|
|
Most recent articles on Levobupivacaine Most cited articles on Levobupivacaine |
|
Media |
|
Powerpoint slides on Levobupivacaine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Levobupivacaine at Clinical Trials.gov Trial results on Levobupivacaine Clinical Trials on Levobupivacaine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Levobupivacaine NICE Guidance on Levobupivacaine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Levobupivacaine Discussion groups on Levobupivacaine Patient Handouts on Levobupivacaine Directions to Hospitals Treating Levobupivacaine Risk calculators and risk factors for Levobupivacaine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Levobupivacaine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Levobupivacaine (rINN) (Template:PronEng) is a local anaesthetic drug belonging to the amino amide group. It is the S-enantiomer of bupivacaine. Levobupivacaine hydrochloride is commonly marketed by AstraZeneca under the trade name Chirocaine.
Clinical use
Compared to bupivacaine, levobupivacaine is associated with less vasodilation and has a longer duration of action. It is approximately 13 per cent less potent (by molarity) than racemic bupivacaine.
Indications
Levobupivacaine is indicated for local anaesthesia including infiltration, nerve block, ophthalmic, epidural and intrathecal anaesthesia in adults; and infiltration analgesia in children.
Contraindications
Levobupivacaine is contraindicated for IV regional anaesthesia (IVRA).
Adverse effects
Adverse drug reactions (ADRs) are rare when it is administered correctly. Most ADRs relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, however allergic reactions can rarely occur.
Systemic exposure to excessive quantities of bupivacaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. CNS effects may include CNS excitation (nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, seizures) followed by depression (drowsiness, loss of consciousness, respiratory depression and apnea). Cardiovascular effects include hypotension, bradycardia, arrhythmias, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression. (Rossi, 2006)
References
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Drug
- Local anesthetics