Lesinurad / allopurinol (Duzallo)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF ACUTE RENAL FAILURE
See full prescribing information for complete Boxed Warning.
*Acute renal failure has occurred with lesinurad, one of the components of DUZALLO
|
Overview
Lesinurad / allopurinol (Duzallo) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
RISK OF ACUTE RENAL FAILURE
See full prescribing information for complete Boxed Warning.
*Acute renal failure has occurred with lesinurad, one of the components of DUZALLO
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lesinurad / allopurinol (Duzallo) in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Lesinurad / allopurinol (Duzallo) and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Lesinurad / allopurinol (Duzallo)
| |
| Combination of | |
| Lesinurad | urate transporter inhibitor |
| Allopurinol | xanthine oxidase inhibitor |
| Identifiers | |
| CAS number | ? |
| ATC code | M04 |
| PubChem | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- DUZALLO combines two medications with complementary mechanisms of action for treatment of hyperuricemia associated with gout: lesinurad, a uric acid reabsorption inhibitor, and allopurinol, a xanthine oxidase inhibitor. DUZALLO lowers serum uric acid levels by increasing excretion and inhibiting production of uric acid.
Lesinurad
- Lesinurad reduces serum uric acid levels by inhibiting the function of transporter proteins involved in uric acid reabsorption in the kidney. Lesinurad inhibited the function of two apical transporters responsible for uric acid reabsorption, uric acid transporter 1 (URAT1) and organic anion transporter 4 (OAT4), with IC50 values of 7.3 and 3.7 µM, respectively. URAT1 is responsible for the majority of the reabsorption of filtered uric acid from the renal tubular lumen. OAT4 is a uric acid transporter associated with diuretic-induced hyperuricemia. Lesinurad does not interact with the uric acid reabsorption transporter SLC2A9 (Glut9), located on the basolateral membrane of the proximal tubule cell.
Allopurinol
- Allopurinol acts on purine catabolism, without disrupting the biosynthesis of purines. It reduces the production of uric acid by inhibiting the biochemical reactions immediately preceding its formation. Allopurinol is a structural analogue of the natural purine base, hypoxanthine. It is an inhibitor of xanthine oxidase, the enzyme responsible for the conversion of hypoxanthine to xanthine and of xanthine to uric acid, the end product of purine metabolism in humans. Allopurinol is metabolized to the corresponding xanthine analogue, oxypurinol (alloxanthine), which also is an inhibitor of xanthine oxidase.
- Reutilization of both hypoxanthine and xanthine for nucleotide and nucleic acid synthesis is markedly enhanced when their oxidations are inhibited by allopurinol and oxypurinol. This reutilization does not disrupt normal nucleic acid anabolism because feedback inhibition is an integral part of purine biosynthesis. As a result of xanthine oxidase inhibition, the serum concentration of hypoxanthine plus xanthine in patients receiving allopurinol for treatment of hyperuricemia is usually in the range of 0.3 to 0.4 mg/dL compared to a normal level of approximately 0.15 mg/dL. A maximum of 0.9 mg/dL of these oxypurines has been reported when the serum urate was lowered to less than 2 mg/dL by high doses of allopurinol. These values are far below the saturation levels at which point their precipitation would be expected to occur (above 7 mg/dL).
Structure
Lesinurad
Allopurinol

Pharmacodynamics
Effects on Serum Uric Acid and Urinary Excretion of Uric Acid
Lesinurad
- In gout patients, lesinurad lowered serum uric acid levels and increased renal clearance and fractional excretion of uric acid. Following single and multiple oral doses of lesinurad to gout patients, dose-dependent decreases in serum uric acid levels and increases in urinary uric acid excretion were observed.
Allopurinol
- Allopurinol reduces both the serum and urinary uric acid levels by inhibiting the formation of uric acid.
Effect on Cardiac Repolarization
Lesinurad
- The effect of lesinurad on cardiac repolarization as assessed by the QTc interval was evaluated in normal healthy subjects and gout patients. Lesinurad at doses up to 1600 mg did not demonstrate an effect on the QTc interval.
Allopurinol
- The effect of allopurinol on cardiac repolarization has not been evaluated.
Pharmacokinetics
DUZALLO
- After the administration of DUZALLO with a high-fat breakfast in healthy subjects, the exposure (AUC) for lesinurad and allopurinol were comparable to the fasted state. Compared to the fasted state, the fed state resulted in 46% and 18% reductions and 2.5 hours and 1.75 hours delays, in the peak (Cmax) concentrations of lesinurad and allopurinol, respectively. This effect of food is not considered to be clinically meaningful. Oxypurinol Cmax and AUC were similar in the fed and fasted states.
Absorption
Lesinurad
- The absolute bioavailability of lesinurad as monotherapy is approximately 100%. Lesinurad is rapidly absorbed after oral administration. Following administration of a single dose of a lesinurad tablet in either fed or fasted state, Cmax was attained within 1 to 4 hours. Cmax and AUC exposures of lesinurad increased proportionally with single doses of lesinurad from 5 to 1200 mg. Administration with a high-fat meal (800 to 1000 calories with 50% of calories being derived from fat content) decreases Cmax by up to 18% but does not alter AUC as compared with fasted state. In clinical trials, lesinurad was administered with food.
Allopurinol
- Allopurinol is approximately 90% absorbed from the gastrointestinal tract. Peak plasma levels generally occur at 1.5 hours and 4.5 hours for allopurinol and oxypurinol, respectively. After a single oral dose of 300 mg allopurinol, maximum plasma levels of about 3 mcg/mL of allopurinol and 6.5 mcg/mL of oxypurinol are produced.
Distribution
Lesinurad
- Lesinurad is extensively bound to proteins in plasma (greater than 98%), mainly to albumin. Plasma protein binding of lesinurad is not meaningfully altered in patients with renal or hepatic impairment. The mean steady state volume of distribution of lesinurad was approximately 20 L following intravenous dosing of lesinurad.
Elimination
Lesinurad
- The elimination half-life (t½) of lesinurad is approximately 5 hours. Lesinurad does not accumulate following multiple doses. The total body clearance is approximately 6 L/hr.
Allopurinol
- Because of its rapid oxidation to oxypurinol and a renal clearance rate approximately that of glomerular filtration rate, allopurinol has a plasma half-life of about 1 to 2 hours. Oxypurinol, however, has a longer plasma elimination half-life (approximately 26 hours) and therefore effective xanthine oxidase inhibition is maintained over a 24-hour period with single daily doses of allopurinol. Whereas allopurinol is cleared essentially by glomerular filtration, oxypurinol is reabsorbed in the kidney tubules in a manner similar to the reabsorption of uric acid.
Metabolism
Lesinurad
- Lesinurad undergoes oxidative metabolism mainly via the polymorphic cytochrome P450 CYP2C9 enzyme. Plasma exposure of metabolites is minimal (<10% of unchanged lesinurad). Metabolites are not known to contribute to the uric acid lowering effects of lesinurad. A transient oxide metabolite is rapidly eliminated by microsomal epoxide hydrolase in the liver and not detected in plasma.
- Patients who are CYP2C9 poor metabolizers are deficient in CYP2C9 enzyme activity. A cross-study pharmacogenomic analysis assessed the association between CYP2C9 polymorphism and lesinurad exposure in patients receiving single or multiple doses of lesinurad at 200 mg, 400 mg or 600 mg. At the 400 mg dose, lesinurad exposure was approximately 1.8-fold higher in CYP2C9 poor metabolizers (i.e., subjects with CYP2C9 *2/*2 [N=1], and *3/*3 [N=1] genotype) compared to CYP2C9 extensive metabolizers (i.e., CYP2C9 *1/*1 [N=41] genotype). Use with caution in CYP2C9 poor metabolizers, and in patients taking moderate inhibitors of CYP2C9.
Allopurinol
- Allopurinol is metabolized to the corresponding xanthine analogue, oxypurinol (alloxanthine), which also is an inhibitor of xanthine oxidase.
Excretion
Lesinurad
- Within 7 days following single dosing of radiolabeled lesinurad, 63% of administered radioactive dose was recovered in urine and 32% of administered radioactive dose was recovered in feces. Most of the radioactivity recovered in urine (> 60% of dose) occurred in the first 24 hours. Unchanged lesinurad in urine accounted for approximately 30% of the dose.
Allopurinol
- Approximately 20% of the ingested allopurinol is excreted in the feces. The remainder is primarily metabolized to oxypurinol.
SPECIFIC POPULATIONS
Renal Impairment
- A dedicated renal impairment study was not conducted with DUZALLO.
Lesinurad
- Two dedicated studies were performed to assess pharmacokinetics (PK) of lesinurad in renal impairment (classified using the Cockcroft-Gault formula) subjects. In both studies, Cmax was comparable in renal impairment subjects compared to healthy subjects. Study 1 was a single-dose, open-label study evaluating the PK of lesinurad 200 mg in subjects with mild (eCLcr 60 to less than 90 mL/min) and moderate renal impairment (eCLcr 30 to less than 60 mL/min) compared to healthy subjects. Compared to healthy subjects (N=6; eCLcr greater than or equal to 90 mL/min), plasma AUC of lesinurad was increased by approximately 30% and 73% in subjects with mild (N=8) and moderate (N=10) renal impairment, respectively. Study 2 was a single-dose, open-label study evaluating the PK of lesinurad 400 mg in subjects with moderate and severe renal impairment (eCLcr less than 30 mL/min) compared to healthy subjects. Compared to healthy subjects (N=6), plasma AUC of lesinurad was increased by approximately 50% and 113% in subjects with moderate (N=6) and severe (N=6) renal impairment, respectively.
Allopurinol
- Allopurinol and its metabolites are primarily eliminated by the kidney. Impairment of renal function may lead to retention of the drug and its metabolites with consequent prolongation of action. Patients with reduced renal function require lower doses of allopurinol than those with normal renal function.
Hepatic Impairment
- A dedicated hepatic impairment study was not conducted with DUZALLO.
Lesinurad
- Following administration of a single dose of lesinurad at 400 mg in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, lesinurad Cmax was comparable and lesinurad AUC was 7% and 33% higher, respectively, compared to individuals with normal hepatic function. There is no clinical experience in patients with severe (Child-Pugh class C) hepatic impairment.
Allopurinol
- No information is available in patients with hepatic impairment.
Effect of Age, Gender, Race and Ethnicity on Pharmacokinetics
Lesinurad
- Based on the population PK analysis, age, gender, race and ethnicity do not have a clinically meaningful effect on the PK of lesinurad.
Allopurinol
- No information is available for age, gender, race, or ethnicity effects on the PK of allopurinol.
Pediatric Use
Lesinurad
- Studies characterizing the PK of lesinurad in pediatric patients have not been conducted.
Allopurinol
- No data on the PK of allopurinol in pediatric patients are available.
Drug-Drug Interactions
- Pharmacokinetic drug interaction studies with DUZALLO have not been performed; however, such studies have been conducted with the individual components lesinurad and allopurinol.
Lesinurad
- Based on in vitro data, lesinurad is a substrate for CYP2C9, OAT1 and OAT3; however, no clinical studies have been conducted with OAT1 and OAT3 inhibitors (e.g., probenecid).
Effects of Other Drugs on Lesinurad
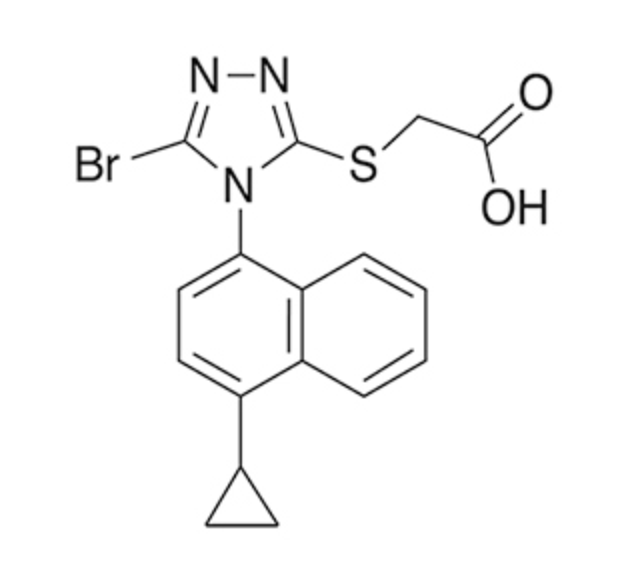
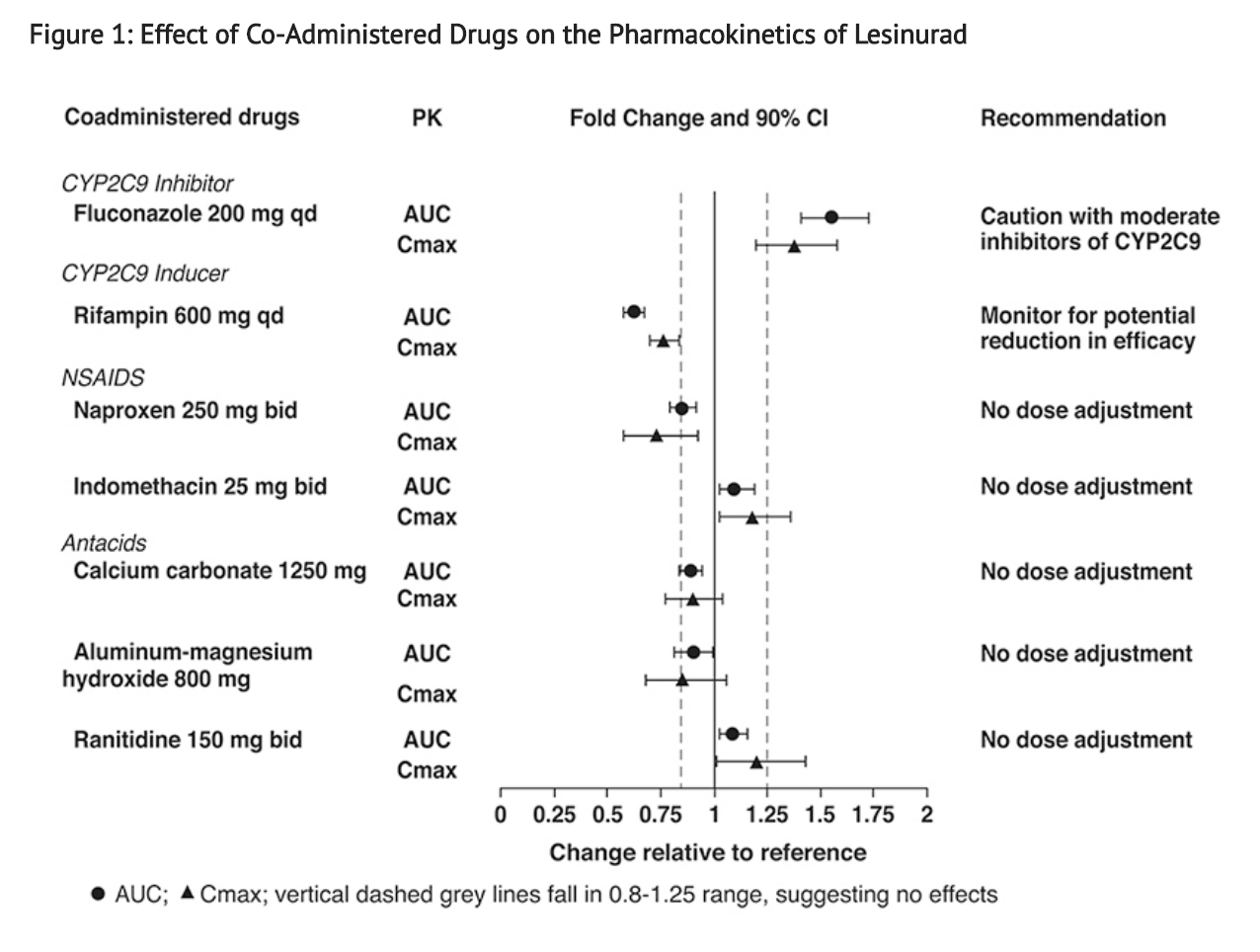
- Figure 1 shows the effect of co-administered drugs on the PK of lesinurad.
Effects of Lesinurad on Other Drugs
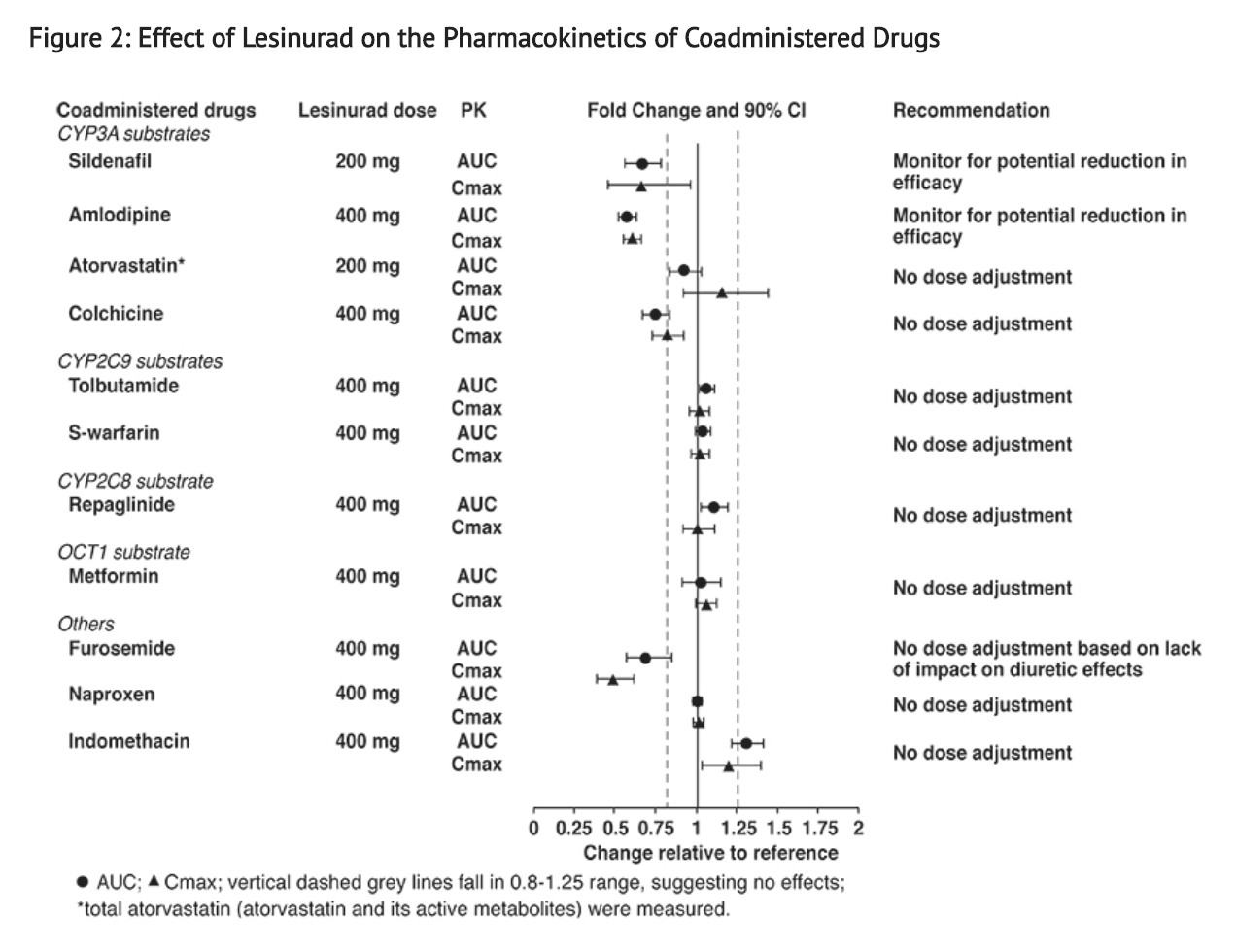
- Lesinurad is a weak inducer of CYP3A and has no relevant effect on any other CYP enzyme for induction (CYP1A2, CYP2C8, CYP2C9, CYP2B6, or CYP2C19) or inhibition (CYP1A2, CYP2B6, CYP2D6, CYP2C8, CYP2C9, CYP2C19, or CYP3A4).
- Based on in vitro studies, lesinurad is an inhibitor of OATP1B1, OCT1, OAT1, and OAT3; however, lesinurad is not an in vivo inhibitor of these transporters. In vivo drug interaction studies indicate that lesinurad does not decrease the renal clearance of furosemide (substrate of OAT1/3), or affect the exposure of atorvastatin (substrate of OATP1B1) or metformin (substrate of OCT1). Based on in vitro studies, lesinurad has no relevant effect on P-glycoprotein.
- Figure 2 shows the effect of lesinurad on co-administered drugs.
Effect of Allopurinol on Mercaptopurine
- Allopurinol inhibits the enzymatic oxidation of mercaptopurine, the sulfur-containing analogue of hypoxanthine, to 6-thiouric acid. This oxidation, which is catalyzed by xanthine oxidase, inactivates mercaptopurine. Hence, the inhibition of such oxidation by allopurinol may result in as much as a 75% reduction in the therapeutic dose requirement of mercaptopurine when the two compounds are given together.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with DUZALLO; however, studies were conducted with its individual components, lesinurad and allopurinol, as described below.
Lesinurad
- The carcinogenic potential of lesinurad was evaluated in a 2-year study conducted in Sprague-Dawley rats and a 26-week study in TgRasH2 mice. No evidence of tumorigenicity was observed in male or female rats at oral doses up to 200 mg/kg/day (approximately 35 times the MRHD on an AUC basis). No evidence of tumorigenicity was observed in TgRasH2 mice at oral doses up to 125 and 250 mg/kg/day in male and female mice, respectively.
- Lesinurad tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo rat bone marrow micronucleus assay.
- Fertility and reproductive performance were unaffected in male or female rats that received lesinurad at oral doses up to 300 mg/kg/day (approximately 15 times the MRHD on a mg/m2 basis).
Allopurinol
- No evidence of tumorigenicity was observed in male or female mice or rats that received oral allopurinol for the majority of their life spans (greater than 88 weeks) at doses up to 20 mg/kg/day (0.3 and 0.6 times the MRHD on a mg/m2 basis in mice and rats respectively).
- Allopurinol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo rat bone marrow micronucleus assay.
- Oral allopurinol doses of 20 mg/kg/day had no effect on male or female fertility in rats or rabbits (approximately 0.6 or 1.3 times the MRHD on a mg/m2 basis, respectively).
Clinical Studies
- Lesinurad in combination with allopurinol has been studied in hyperuricemic gout patients who have not achieved target serum uric acid levels with allopurinol alone.
- There have been no phase 3 clinical trials with DUZALLO. Bioequivalence of DUZALLO to co-administered lesinurad and allopurinol was demonstrated, and efficacy of the combination of allopurinol and lesinurad has been demonstrated in two phase 3 studies (Study 1 and 2).
Lesinurad Add-On to Allopurinol in Inadequate Responders
- Both studies were 12-month multicenter, randomized, double-blind, placebo-controlled clinical studies in adult patients with hyperuricemia and gout. Patients received prophylaxis for gout flares with colchicine or non-steroidal anti-inflammatory drugs (NSAIDs) during the first 5 months of study treatment. Lesinurad 200 mg and 400 mg once daily in combination with allopurinol were studied. Lesinurad 200 mg is the only recommended lesinurad dose, and is the dose combined with allopurinol in DUZALLO.
- Study 1 and Study 2 enrolled patients with gout who were on a stable dose of allopurinol of at least 300 mg (or 200 mg for moderate renal impairment), had a serum uric acid > 6.5 mg/dL, and reported at least 2 gout flares in the prior 12 months. Mean years since gout diagnosis were 12 years. More than half of the patients (61%) had mild or moderate renal impairment and 19% of the patients had tophi. Patients were randomized 1:1:1 to receive lesinurad 200 mg, lesinurad 400 mg, or placebo once daily; all were to continue on their stable allopurinol dose. The majority of patients in these studies received daily allopurinol doses of 200 mg or 300 mg, corresponding to the allopurinol doses contained in DUZALLO. The average dose of allopurinol in the studies was 310 mg (range: 200-900 mg).
- As shown in Table 5, lesinurad 200 mg in combination with allopurinol was superior to allopurinol alone in lowering serum uric acid to less than 6 mg/dL at Month 6.

- The estimated effect of lesinurad 200 mg on serum uric acid in the subgroup of patients taking thiazide diuretics at baseline was similar to the estimated effect in the overall population. The estimated effect was also similar in the subgroup of patients taking low-dose aspirin at baseline.
- As shown in Figure 3, reduction in average serum uric acid levels to < 6 mg/dL was noted for lesinurad 200 mg in combination with allopurinol at the Month 1 visit and was maintained throughout the 12-month studies.

Gout Flares
- In Study 1 and Study 2, the rates of gout flare requiring treatment from the end of Month 6 to the end of Month 12 were not statistically different between lesinurad 200 mg in combination with allopurinol compared with allopurinol alone.
Use in Patients With Renal Impairment
- The estimated differences between lesinurad and placebo in the proportions of patients achieving target serum uric acid levels in the renal impairment subgroups were largely consistent with the results in the overall population in the studies. However, there were limited data in patients with eCLcr less than 45 mL/min and there was a trend toward decreasing magnitude of effect with decreasing renal function. Based on integrated data from Study 1 and Study 2, the estimated difference between lesinurad 200 mg in combination with allopurinol and allopurinol alone in the proportion achieving serum uric acid < 6.0 mg/dL at Month 6 was 10% (95% CI: -17, 37) in those with eCLcr less than 45 mL/min as compared with 27% (95% CI: 9, 45) in the 45 to less than 60 mL/min subgroup and 30% (95% CI: 23, 37) in the 60 mL/min or greater subgroup.
How Supplied
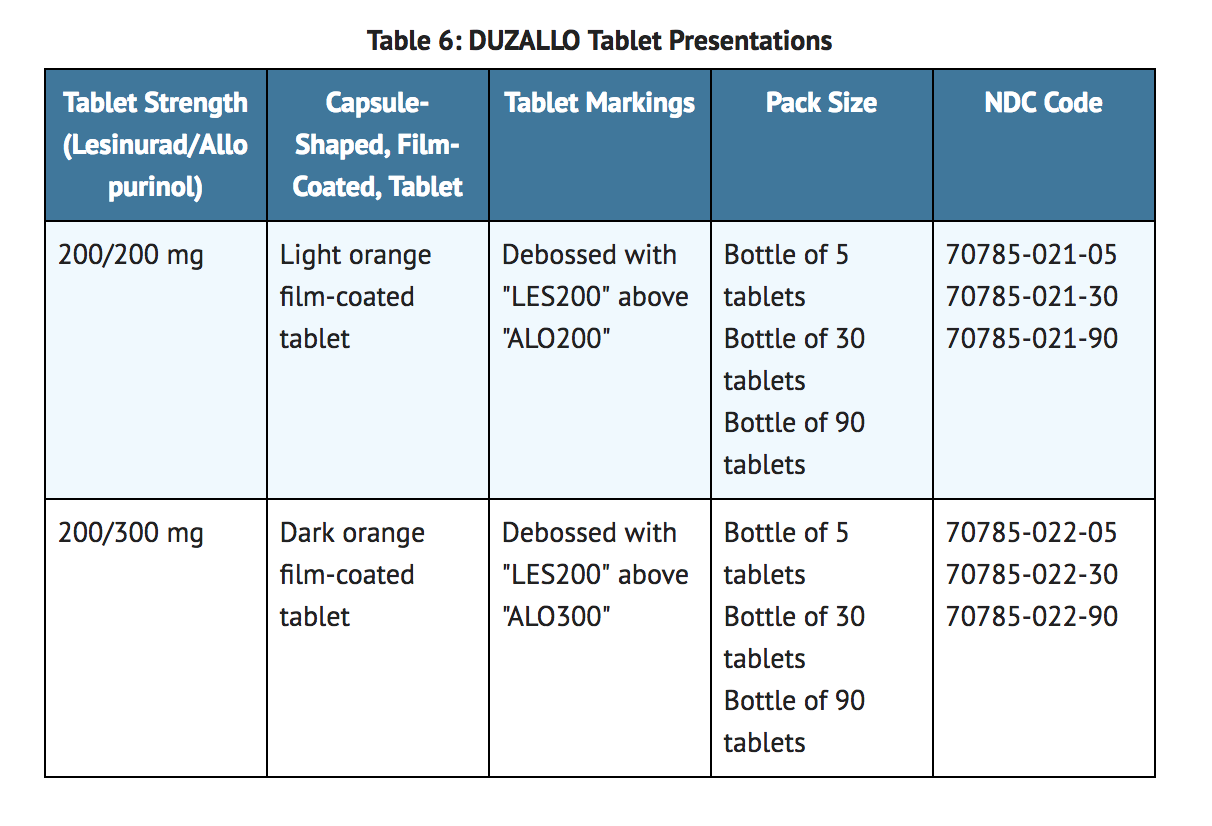
- DUZALLO tablets have markings on one side, are plain on the reverse side and are available in the strengths and packages listed in Table 6.
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F).
Images
Drug Images
{{#ask: Page Name::Lesinurad / allopurinol (Duzallo) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Lesinurad / allopurinol (Duzallo) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Administration
- Advise patients:
- To take one tablet of DUZALLO in the morning with food and water.
- Not to take a missed dose of DUZALLO later in the day, but to wait to take DUZALLO on the next day, and not to double the dose.
- Not to take DUZALLO with ZURAMPIC (lesinurad).
- To stay well hydrated (e.g., 2 liters of liquid per day).
Renal Events
- Inform patients that renal events including transient increases in blood creatinine level and acute renal failure have occurred in some patients who take DUZALLO. Advise patients that periodic monitoring of blood creatinine levels is recommended.
Serious Skin Reactions
- Inform patients that DUZALLO may increase the risk of serious skin side effects such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching and should ask for medical advice when observing any indicative signs or symptoms. Advise patients to stop the drug immediately if they develop any type of rash and seek medical attention.
Gout Flares
- Inform patients that gout flares may occur after initiation of DUZALLO and of the importance of taking gout flare prophylaxis medication to help prevent gout flares. Advise patients not to discontinue DUZALLO if a gout flare occurs during treatment.
Concomitant Use with Other Medications
- Inform patients that use of DUZALLO may increase the risks associated with taking other medications (i.e., coumarin anticoagulants, mercaptopurine, azathioprine and thiazide diuretics) and they should follow the instructions of their healthcare provider.
Ability to Perform Complex Tasks
- Patients should be informed that drowsiness has been reported in patients taking allopurinol. Patients should be alerted to the need for due caution when engaging in activities where alertness is mandatory.

Precautions with Alcohol
Alcohol-Lesinurad / allopurinol (Duzallo) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Duzallo
Look-Alike Drug Names
There is limited information regarding Lesinurad / allopurinol (Duzallo) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.