Lesinurad / allopurinol (Duzallo)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF ACUTE RENAL FAILURE
See full prescribing information for complete Boxed Warning.
*Acute renal failure has occurred with lesinurad, one of the components of DUZALLO
|
Overview
Lesinurad / allopurinol (Duzallo) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
RISK OF ACUTE RENAL FAILURE
See full prescribing information for complete Boxed Warning.
*Acute renal failure has occurred with lesinurad, one of the components of DUZALLO
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lesinurad / allopurinol (Duzallo) in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Lesinurad / allopurinol (Duzallo) and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Lesinurad / allopurinol (Duzallo)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
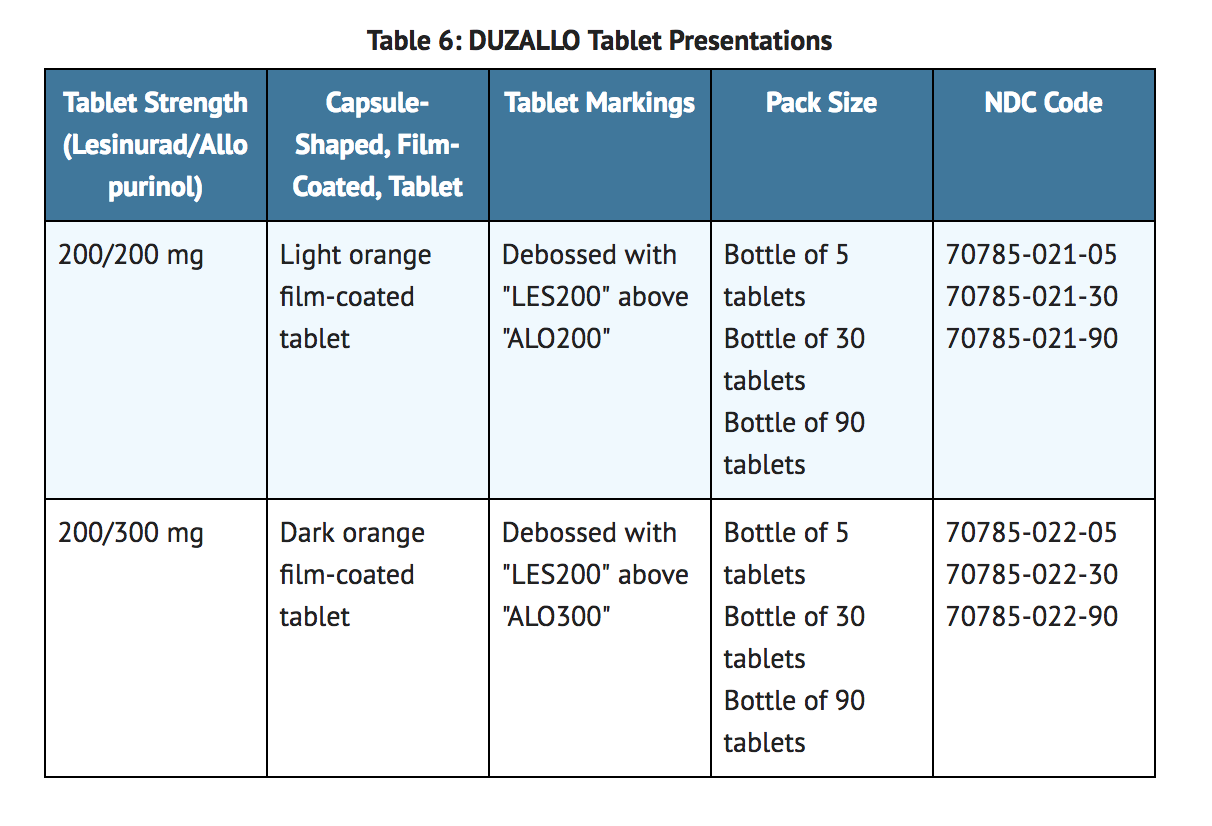
- DUZALLO tablets have markings on one side, are plain on the reverse side and are available in the strengths and packages listed in Table 6.
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F).
Images
Drug Images
{{#ask: Page Name::Lesinurad / allopurinol (Duzallo) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Lesinurad / allopurinol (Duzallo) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Administration
- Advise patients:
- To take one tablet of DUZALLO in the morning with food and water.
- Not to take a missed dose of DUZALLO later in the day, but to wait to take DUZALLO on the next day, and not to double the dose.
- Not to take DUZALLO with ZURAMPIC (lesinurad).
- To stay well hydrated (e.g., 2 liters of liquid per day).
Renal Events
- Inform patients that renal events including transient increases in blood creatinine level and acute renal failure have occurred in some patients who take DUZALLO. Advise patients that periodic monitoring of blood creatinine levels is recommended.
Serious Skin Reactions
- Inform patients that DUZALLO may increase the risk of serious skin side effects such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching and should ask for medical advice when observing any indicative signs or symptoms. Advise patients to stop the drug immediately if they develop any type of rash and seek medical attention.
Gout Flares
- Inform patients that gout flares may occur after initiation of DUZALLO and of the importance of taking gout flare prophylaxis medication to help prevent gout flares. Advise patients not to discontinue DUZALLO if a gout flare occurs during treatment.
Concomitant Use with Other Medications
- Inform patients that use of DUZALLO may increase the risks associated with taking other medications (i.e., coumarin anticoagulants, mercaptopurine, azathioprine and thiazide diuretics) and they should follow the instructions of their healthcare provider.
Ability to Perform Complex Tasks
- Patients should be informed that drowsiness has been reported in patients taking allopurinol. Patients should be alerted to the need for due caution when engaging in activities where alertness is mandatory.

Precautions with Alcohol
Alcohol-Lesinurad / allopurinol (Duzallo) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Duzallo
Look-Alike Drug Names
There is limited information regarding Lesinurad / allopurinol (Duzallo) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.