Kumada coupling
Overview
A Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry between a alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalysed by nickel or palladium. This reaction is relevant to organic synthesis because it gives access to styrene compounds. The reaction type was reported independently by two groups in 1972.
Development
This method builds on earlier work done by Tamura & Kochi in 1971 on couplings of Grignards with catalytical amounts of other metal halide catalysts than nickel for instance silver [1]. Stoichometric Grignard couplings and Grignard homo-couplings have been known well before that time.
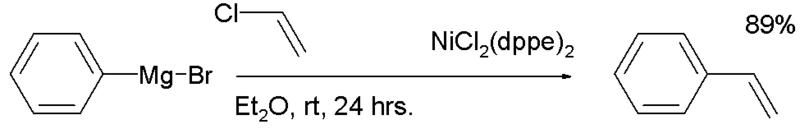
The first report by the Kumada group [2] described the reaction of a Grigard reagent for instance phenylmagnesium bromide with an aryl or vinyl chloride such as vinyl chloride to the coupled product (styrene) catalyzed by a nickel chloride with two dppe ligands (NiCl2dppe2):
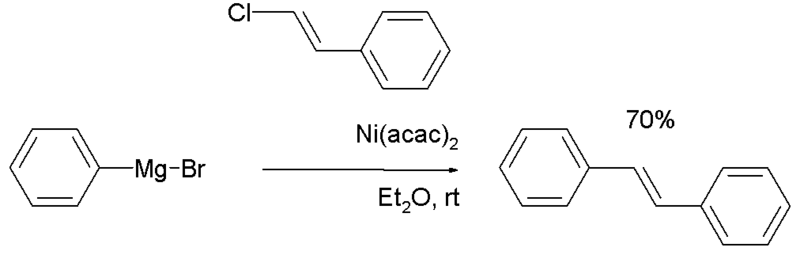
In the 1972 Corriu report [3], beta-bromostyrene is reacted with phenylmagnesium bromide to trans-stilbene in diethylether also with nickel catalysts notably Nickel(II) acetylacetonate.
Palladium was introduced to this chemistry in 1975 by Murahashi [4] when Tetrakis(triphenylphosphine)palladium(0) was found to catalyze the reaction of (Z)-bromostyrene with methylmagnesium iodide to (Z)-propenylbenzene. With the far more reactive methyl lithium, the palladium catalyst is not recycled fast enough and an elimination reaction to the alkyne predominates.
Reaction mechanism
The Nickel reaction mechanism for Ni(II) catalysts is a sequence of several steps [5]:
- Transmetallation: the dihalonickel catalyst reacts with the Grignard RMgX to a diorganonickel intermediate NiR2L2 and dihalonickel NiX2
- Reductive elimination: reaction of NiR2L2 with the organohalide R'X forms the coupled product R-R together with the nickelorganohalide NiR'XL2. In the overall reaction this step is negligible because the active nickel compound is formed in catalytical amounts
- Transmetallation: in the first step of the catalytic cycle nickelorganohalide NiR'XL2 reacts with another equivalent of RMgX to mixed diorganonickel NiRR'L2 compound and dihalonickel NiX2
- trans-cis isomerization: with substrates trans-dichloroethylene and phenylmagnesium chloride, the resulting stilbene is enriched in the cis isomer.
- Coordination: a new equivalent of organohalide R'-X adds face-on to the mixed dihalonickel complex
- Oxidative addition: The cross-coupled product R-R' is released with regeneration of nickelorganohalide NiR'XL2
The main steps in the mechanism for Ni(0) or Pd(0) catalysts are oxidative addition of the organohalide, transmetallation of the Grignard and reductive elimination.
Scope
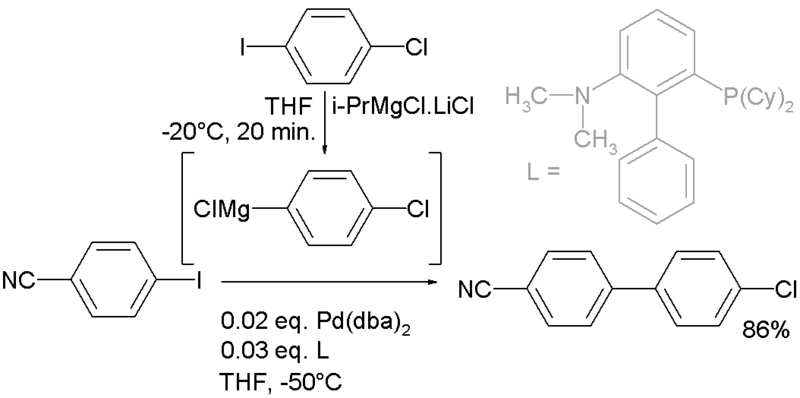
Recently the scope of this reaction was extended to aryl-aryl couplings with improved functional group tolerance [6]:
In this particular reaction the Grignard is prepared in situ by I/Mg exchange between an aryl iodide and isopropylmagnesium chloride / lithium chloride [7].
References
- ↑ Mechanism of the silver-catalyzed reaction of Grignard reagents with alkyl halides Jay K. Kochi and Masuhiko Tamura J. Am. Chem. Soc.; 1971; 93(6) pp 1483 - 1485; doi:10.1021/ja00735a028
- ↑ Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes Kohei Tamao, Koji Sumitani, Makoto Kumada J. Am. Chem. Soc.; 1972; 94(12); 4374-4376. doi:10.1021/ja00767a074
- ↑ Activation of Grignard Reagents by Transition-metal Complexes. A New and Simple Synthesis of trans-Stilbenes and Polyphenyls Corriu, R. J. P.; Masse, J. P. J. Chem. Soc., Chem. Commun. 1972,144.
- ↑ The reaction of σ-vinylpalladium complexes with alkyllithiums. Stereospecific syntheses of olefins from vinyl halides and alkyllithiums Journal of Organometallic Chemistry, Volume 91, Issue 2, 27 May 1975, Pages C39-C42 Masaaki Yamamura, Ichiro Moritani and Shun-Ichi Murahashi doi:10.1016/S0022-328X(00)89636-9
- ↑ Strategic Applications of Named Reactions in Organic Synthesis Laszlo Kurti, Barbara Czako Academic Press (March 4, 2005) ISBN 0-12-429785-4
- ↑ Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents Ruben Martin and Stephen L. Buchwald J. Am. Chem. Soc.; 2007; 129(13) pp 3844 - 3845; (Communication) doi:10.1021/ja070830d
- ↑ A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroarylmagnesium Compounds from Organic Bromides Arkady Krasovskiy, Paul Knochel Angewandte Chemie International Edition Volume 43, Issue 25 , Pages 3333 - 3336 2004 doi:10.1002/anie.200454084