Isometheptene, caffeine and acetaminophen: Difference between revisions
No edit summary |
No edit summary |
||
| Line 73: | Line 73: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = XVQUOJBERHHONY-UHFFFAOYSA-N | | StdInChIKey = XVQUOJBERHHONY-UHFFFAOYSA-N | ||
}} | }} | ||
|howSupplied=*Isometheptene, Caffeine, and Acetaminophen Tablets are supplied as white tablets debossed “130” on one side, and scored on the opposite side. Available in bottles of 50 tablets, NDC 50967-620-50. | |howSupplied=*Isometheptene, Caffeine, and Acetaminophen Tablets are supplied as white tablets debossed “130” on one side, and scored on the opposite side. Available in bottles of 50 tablets, NDC 50967-620-50. | ||
Latest revision as of 19:24, 27 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
Hepatotoxicity:
|
Overview
Isometheptene, caffeine and acetaminophen is an analgesic that is FDA approved for the treatment of tension and vascular headaches. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hepatotoxicity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Migraine Headache
- Dosage: The usual adult dosage is two tablets at once, followed by one tablet every hour until relieved, up to 5 tablets within a twelve hour period.
Tensional Headache
- Dosage: The usual adult dosage is one or two tablets every four hours up to 8 tablets a day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isometheptene, caffeine and acetaminophen in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Isometheptene, caffeine and acetaminophen in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Isometheptene, caffeine and acetaminophen FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isometheptene, caffeine and acetaminophen in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Isometheptene, caffeine and acetaminophen in pediatric patients.
Contraindications
- This Product is contraindicated in Glaucoma and/or severe cases of renal disease, hypertension, organic heart disease, hepatic disease, and in those patients who are on monoamine oxidase inhibitor (MAOI) therapy.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
Hepatotoxicity:
|
Hepatotoxicity
- Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products. The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
- Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4,000 milligrams of acetaminophen per day, even if they feel well.
Hypersensitivity/Anaphylaxis
- There have been post-marketing reports of hypersensitivity and anaphylaxis associated with the use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue Isometheptene, Caffeine, and Acetaminophen Tablets immediately and seek medical care if they experience these symptoms. Do not prescribe Isometheptene, Caffeine, and Acetaminophen Tablets for patients with acetaminophen allergy.
Adverse Reactions
Clinical Trials Experience
- Hypersensitive patients have shown rash and transient dizziness, this can be eliminated by reducing dosage.
Postmarketing Experience
There is limited information regarding Isometheptene, caffeine and acetaminophen Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Isometheptene, caffeine and acetaminophen Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Isometheptene, caffeine and acetaminophen in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Isometheptene, caffeine and acetaminophen in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Isometheptene, caffeine and acetaminophen during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in geriatric settings.
Gender
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen with respect to specific gender populations.
Race
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Isometheptene, caffeine and acetaminophen in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Isometheptene, caffeine and acetaminophen in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Isometheptene, caffeine and acetaminophen Administration in the drug label.
Monitoring
There is limited information regarding Isometheptene, caffeine and acetaminophen Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Isometheptene, caffeine and acetaminophen and IV administrations.
Overdosage
Following an acute overdosage, toxicity may result
- Acetaminophen – In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Pharmacology
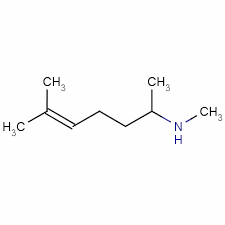
| |
Isometheptene, caffeine and acetaminophen
| |
| Systematic (IUPAC) name | |
| N,6-dimethylhept-5-en-2-amine | |
| Identifiers | |
| CAS number | |
| ATC code | A03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 141.254 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Isometheptene, caffeine and acetaminophen Mechanism of Action in the drug label.
Structure
There is limited information regarding Isometheptene, caffeine and acetaminophen Structure in the drug label.
Pharmacodynamics
There is limited information regarding Isometheptene, caffeine and acetaminophen Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Isometheptene, caffeine and acetaminophen Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Isometheptene, caffeine and acetaminophen Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Isometheptene, caffeine and acetaminophen Clinical Studies in the drug label.
How Supplied
- Isometheptene, Caffeine, and Acetaminophen Tablets are supplied as white tablets debossed “130” on one side, and scored on the opposite side. Available in bottles of 50 tablets, NDC 50967-620-50.
Storage
- Store at 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Avoid exposure to heat.
Images
Drug Images
{{#ask: Page Name::Isometheptene, caffeine and acetaminophen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

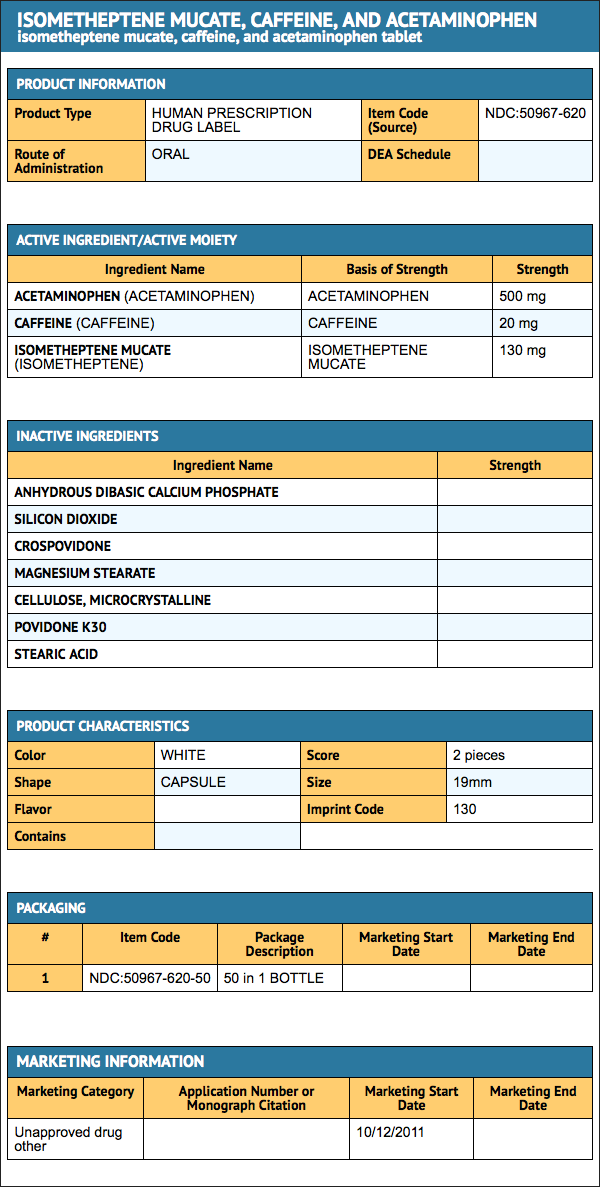
{{#ask: Label Page::Isometheptene, caffeine and acetaminophen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Isometheptene, caffeine and acetaminophen Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Isometheptene, caffeine and acetaminophen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Isometheptene, caffeine and acetaminophen Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Isometheptene, caffeine and acetaminophen Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.