Ioflupane i-123
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ioflupane i-123 is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Ioflupane i-123 FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ioflupane i-123 in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioflupane i-123 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ioflupane i-123 FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ioflupane i-123 in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioflupane i-123 in pediatric patients.
Contraindications
DaTscan is contraindicated in patients with known hypersensitivity to the active substance or to any of the excipients, or to iodine.
Warnings
5.1 Hypersensitivity Reactions Hypersensitivity reactions have been reported following DaTscan administration [see ADVERSE REACTIONS (6.2)]. The reactions have generally consisted of skin erythema and pruritis and have either resolved spontaneously or following the administration of corticosteroids and anti-histamines. Prior to administration, question the patient for a history of prior reactions to DaTscan. If the patient is known or strongly suspected of having had a hypersensitivity reaction to DaTscan, the decision to administer DaTscan should be based upon an assessment of the expected benefits compared to the potential hypersensitivity risks. Have anaphylactic and hypersensitivity treatment measures available prior to DaTscan administration and, following administration, observe patients for symptoms or signs of a hypersensitivity reaction.
5.2 Thyroid Accumulation The DaTscan injection may contain up to 6% of free iodide (iodine 123). To decrease thyroid accumulation of iodine 123, block the thyroid gland before administration of DaTscan [see DOSAGE AND ADMINISTRATION (2.2)]. Avoid the use of Potassium Iodide Oral Solution or Lugol's Solution in patients who are sensitive to such products. Failure to block thyroid uptake of iodine 123 may result in an increased long term risk for thyroid neoplasia.
Adverse Reactions
Clinical Trials Experience
6.1 Clinical Study Experience The data from clinical studies reflect exposure to DaTscan in 942 subjects with a mean age of 66 years (range 25 to 90 years). Among these subjects, 42% were women and 99% Caucasian. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of DaTscan cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical trials, no serious adverse reactions were reported. Other adverse reactions occurred at a rate of 1% or less and the reported events consisted of headache, nausea, vertigo, dry mouth or dizziness. These reactions were of mild to moderate severity.
Postmarketing Experience
6.2 Postmarketing Experience Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. In the postmarketing experience, hypersensitivity reactions have been reported. The reactions generally related to rash and pruritis within minutes of DaTscan administration. The reactions either resolved spontaneously or following the administration of corticosteroids and antihistamines. Injection site pain has also been reported.
Drug Interactions
The ioflupane within DaTscan binds to the dopamine transporter. Drugs that bind to the dopamine transporter with high affinity may interfere with the image obtained following DaTscan administration. These potentially interfering drugs consist of: amoxapine, amphetamine, benztropine, bupropion, buspirone, cocaine, mazindol, methamphetamine, methylphenidate, norephedrine, phentermine, phenylpropanolamine, selegiline, and sertraline. Selective serotonin reuptake inhibitors (paroxetine and citalopram) may increase or decrease ioflupane binding to the dopamine transporter. Whether discontinuation of these drugs prior to DaTscan administration may minimize the interference with a DaTscan image is unknown.
The impact of dopamine agonists and antagonists upon DaTscan imaging results has not been established.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C It is not known whether DaTscan can cause fetal harm or increase the risk of pregnancy loss when administered to a pregnant woman. Animal reproductive and developmental toxicity studies have not been conducted with DaTscan. Prior to the administration of DaTscan to women of childbearing potential, assess for the presence of pregnancy. DaTscan should be given to a pregnant woman only if clearly needed.
Like all radiopharmaceuticals, DaTscan has a potential to cause fetal harm. The likelihood of fetal harm depends on the stage of fetal development, and the magnitude of the radionuclide dose. Administration of DaTscan at a dose of 185 MBq (5 mCi) results in an absorbed radiation dose to the uterus of 0.3 rad (3.0 mGy). Radiation doses greater than 15 rad (150 mGy) have been associated with congenital anomalies but doses under 5 rad (50 mGy) generally have not. Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ioflupane i-123 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ioflupane i-123 during labor and delivery.
Nursing Mothers
It is not known whether DaTscan is excreted into human milk. However, iodine 123 is excreted into human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to interrupt nursing after administration of DaTscan or not to administer DaTscan, taking into account the importance of the drug to the mother. Based on the physical half-life of iodine 123 (13.2 hours), nursing women may consider interrupting nursing and pumping and discarding breast milk for 6 days after DaTscan administration in order to minimize risks to a nursing infant.
Pediatric Use
DaTscan is not indicated for use in children. The safety and efficacy of DaTscan have not been established in pediatric patients.
Geriatic Use
In the two principal clinical studies, 45% of the subjects were aged 65 and over. There were no differences in response compared to younger subjects that would require a dose adjustment. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Ioflupane i-123 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ioflupane i-123 with respect to specific racial populations.
Renal Impairment
The effect of renal or hepatic impairment upon DaTscan imaging has not been established. DaTscan is excreted by the kidney and patients with severe renal impairment may have increased radiation exposure and altered DaTscan images.
Hepatic Impairment
The effect of renal or hepatic impairment upon DaTscan imaging has not been established.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ioflupane i-123 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ioflupane i-123 in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ioflupane i-123 Administration in the drug label.
Monitoring
There is limited information regarding Ioflupane i-123 Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ioflupane i-123 and IV administrations.
Overdosage
The clinical consequence of overdose with DaTscan has not been reported. It is unknown whether or not ioflupane is dialyzable. Due to the small quantity of ioflupane in each vial, overdosage with ioflupane is not expected to result in pharmacologic effects. The major risks of overdose relates predominantly to increased radiation exposure, with the long-term risks for neoplasia. In case of overdosage of radioactivity, frequent urination and defecation should be encouraged to minimize radiation exposure to the patient; care should be taken to avoid contamination from the radioactivity eliminated by the patient.
Pharmacology
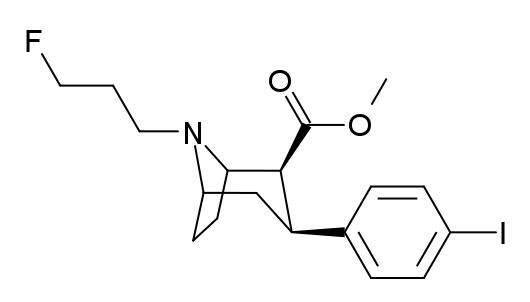 | |
| Clinical data | |
|---|---|
| Synonyms | Ioflupane (FPCIT); [I-123] N-ω-fluoropropyl- 2β-carbomethoxy- 3β-(4-iodophenyl) nortropane |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H23FINO2 |
| Molar mass | 427.285 g/mol |
| 3D model (JSmol) | |
| |
| (verify) | |
Mechanism of Action
There is limited information regarding Ioflupane i-123 Mechanism of Action in the drug label.
Structure
There is limited information regarding Ioflupane i-123 Structure in the drug label.
Pharmacodynamics
There is limited information regarding Ioflupane i-123 Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Ioflupane i-123 Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Ioflupane i-123 Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ioflupane i-123 Clinical Studies in the drug label.
How Supplied
There is limited information regarding Ioflupane i-123 How Supplied in the drug label.
Storage
There is limited information regarding Ioflupane i-123 Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ioflupane i-123 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ioflupane i-123 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ioflupane i-123 Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ioflupane i-123 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ioflupane i-123 Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ioflupane i-123 Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- Pages with ignored display titles
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link