Indinavir
{{DrugProjectFormSinglePage |authorTag=Alberto Plate [1] |genericName=Indinavir |aOrAn=an |drugClass=antiretroviral agent and protease inhibitor |indicationType=treatment |indication=HIV infection in combination with other antiretroviral agents |adverseReactions=abdominal pain, heartburn, loss of appetite, nausea, taste sense altered, vomiting, neutrophil count abnormal, hyperbilirubinemia and headache |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=The recommended dosage of Indinavir is 800 mg (usually two 400-mg capsules) orally every 8 hours.
- Indinavir must be taken at intervals of 8 hours. For optimal absorption, Indinavir should be administered without food but with water 1 hour before or 2 hours after a meal. Alternatively, Indinavir may be administered with other liquids such as skim milk, juice, coffee, or tea, or with a light meal, e.g., dry toast with jelly, juice, and coffee with skim milk and sugar; or corn flakes, skim milk and sugar. To ensure adequate hydration, it is recommended that adults drink at least 1.5 liters (approximately 48 ounces) of liquids during the course of 24 hours.
Concomitant Therapy
- Delavirdine: Dose reduction of Indinavir to 600 mg every 8 hours should be considered when administering delavirdine 400 mg three times a day.
- Didanosine: If indinavir and didanosine are administered concomitantly, they should be administered at least one hour apart on an empty stomach (consult the manufacturer's product circular for didanosine).
- Itraconazole: Dose reduction of Indinavir to 600 mg every 8 hours is recommended when administering itraconazole 200 mg twice daily concurrently.
- Ketoconazole: Dose reduction of Indinavir to 600 mg every 8 hours is recommended when administering ketoconazole concurrently.
- Rifabutin: Dose reduction of rifabutin to half the standard dose (consult the manufacturer's product circular for rifabutin) and a dose increase of Indinavir to 1000 mg every 8 hours are recommended when rifabutin and Indinavir are coadministered.
Hepatic Insufficiency
- The dosage of Indinavir should be reduced to 600 mg every 8 hours in patients with mild-to-moderate hepatic insufficiency due to cirrhosis.
Nephrolithiasis/Urolithiasis
- In addition to adequate hydration, medical management in patients who experience nephrolithiasis/urolithiasis may include temporary interruption (e.g., 1 to 3 days) or discontinuation of therapy.
|offLabelAdultGuideSupport=====Prophylaxis for Non-Ocuppational Exposure to HIV====
- Dosage[1]:
- Monotherapy: 800 mg every 8 hours
- Combined therapy: If combining with ritonavir, 800 mg indinavir and 100 mg ritonavir every 12 hours or 800 mg indinavir and 200 mg ritonavir every 12 hours
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Indinavir in adult patients. |offLabelPedGuideSupport=====Prophylaxis for Non-Ocuppational Exposure to HIV====
- Dosage[2]:
- Children (3 years to 12 years of age): 450 to 500 mg/m(2) PO q8h
- Adolescents (13 years of age or greater): 800 mg PO 3 q8h
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Indinavir in pediatric patients. |contraindications=*Indinavir is contraindicated in patients with clinically significant hypersensitivity to any of its components.
- Inhibition of CYP3A4 by Indinavir can result in elevated plasma concentrations of the following drugs, potentially causing serious or life-threatening reactions:
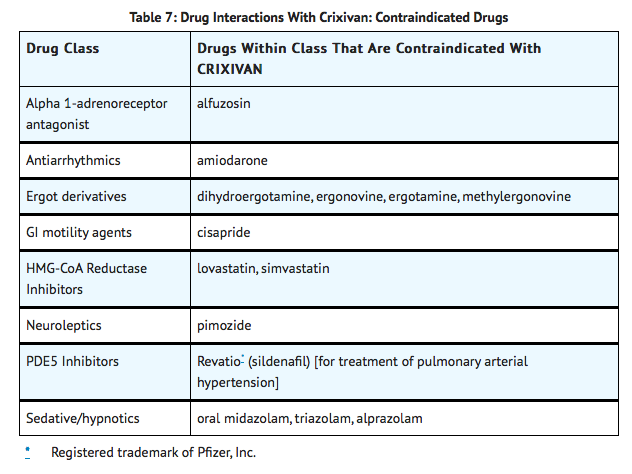
|warnings=ALERT: Find out about medicines that should NOT be taken with Indinavir. This statement is included on the product's bottle label.
Nephrolithiasis/Urolithiasis
Nephrolithiasis/urolithiasis has occurred with Indinavir therapy. The cumulative frequency of nephrolithiasis is substantially higher in pediatric patients (29%) than in adult patients (12.4%; range across individual trials: 4.7% to 34.4%). The cumulative frequency of nephrolithiasis events increases with increasing exposure to Indinavir; however, the risk over time remains relatively constant. In some cases, nephrolithiasis/urolithiasis has been associated with renal insufficiency or acute renal failure, pyelonephritis with or without bacteremia. If signs or symptoms of nephrolithiasis/urolithiasis occur, (including flank pain, with or without hematuria or microscopic hematuria), temporary interruption (e.g., 1-3 days) or discontinuation of therapy may be considered. Adequate hydration is recommended in all patients treated with Indinavir.
Hemolytic Anemia
Acute hemolytic anemia, including cases resulting in death, has been reported in patients treated with Indinavir Once a diagnosis is apparent, appropriate measures for the treatment of hemolytic anemia should be instituted, including discontinuation of Indinavir.
Hepatitis
Hepatitis including cases resulting in hepatic failure and death has been reported in patients treated with Indinavir Because the majority of these patients had confounding medical conditions and/or were receiving concomitant therapy(ies), a causal relationship between Indinavir and these events has not been established.
Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
Drug Interactions
Concomitant use of Indinavir with lovastatin or simvastatin is contraindicated due to an increased risk of myopathy including rhabdomyolysis. Caution should be exercised if Indinavir is used concurrently with atorvastatin or rosuvastatin. Titrate the atorvastatin and rosuvastatin doses carefully and use the lowest necessary dose with Indinavir.
Midazolam is extensively metabolized by CYP3A4. Co-administration with Indinavir with or without ritonavir may cause a large increase in the concentration of this benzodiazepine. No drug interaction study has been performed for the co-administration of Indinavir with benzodiazepines. Based on data from other CYP3A4 inhibitors, plasma concentrations of midazolam are expected to be significantly higher when midazolam is given orally. Therefore Indinavir should not be co-administered with orally administered midazolam, whereas caution should be used with co-administration of Indinavir and parenteral midazolam. Data from concomitant use of parenteral midazolam with other protease inhibitors suggest a possible 3-4 fold increase in midazolam plasma levels. If Indinavir with or without ritonavir is co-administered with parenteral midazolam, it should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered.
Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients receiving indinavir. Coadministration of Indinavir with these medications is expected to substantially increase plasma concentrations of sildenafil, tadalafil, and vardenafil and may result in an increase in adverse events, including hypotension, visual changes, and priapism, which have been associated with sildenafil, tadalafil, and vardenafil.
Concomitant use of Indinavir and St. John's wort (Hypericum perforatum) or products containing St. John's wort is not recommended. Coadministration of Indinavir and St. John's wort has been shown to substantially decrease indinavir concentrations and may lead to loss of virologic response and possible resistance to Indinavir or to the class of protease inhibitors. |clinicalTrials=====Clinical Trials in Adults====
- Nephrolithiasis/urolithiasis, including flank pain with or without hematuria (including microscopic hematuria), has been reported in approximately 12.4% (301/2429; range across individual trials: 4.7% to 34.4%) of patients receiving Indinavir at the recommended dose in clinical trials with a median follow-up of 47 weeks (range: 1 day to 242 weeks; 2238 patient-years follow-up). The cumulative frequency of nephrolithiasis events increases with duration of exposure to Indinavir however, the risk over time remains relatively constant. Of the patients treated with Indinavir who developed nephrolithiasis/urolithiasis in clinical trials during the double-blind phase, 2.8% (7/246) were reported to develop hydronephrosis and 4.5% (11/246) underwent stent placement. Following the acute episode, 4.9% (12/246) of patients discontinued therapy.
- Asymptomatic hyperbilirubinemia (total bilirubin ≥2.5 mg/dL), reported predominantly as elevated indirect bilirubin, has occurred in approximately 14% of patients treated with Indinavir. In <1% this was associated with elevations in ALT or AST.
- Hyperbilirubinemia and nephrolithiasis/urolithiasis occurred more frequently at doses exceeding 2.4 g/day compared to doses ≤2.4 g/day.
Clinical adverse experiences reported in ≥2% of patients treated with Indinavir alone, Indinavir in combination with zidovudine or zidovudine plus lamivudine, zidovudine alone, or zidovudine plus lamivudine are presented in TABLE 10
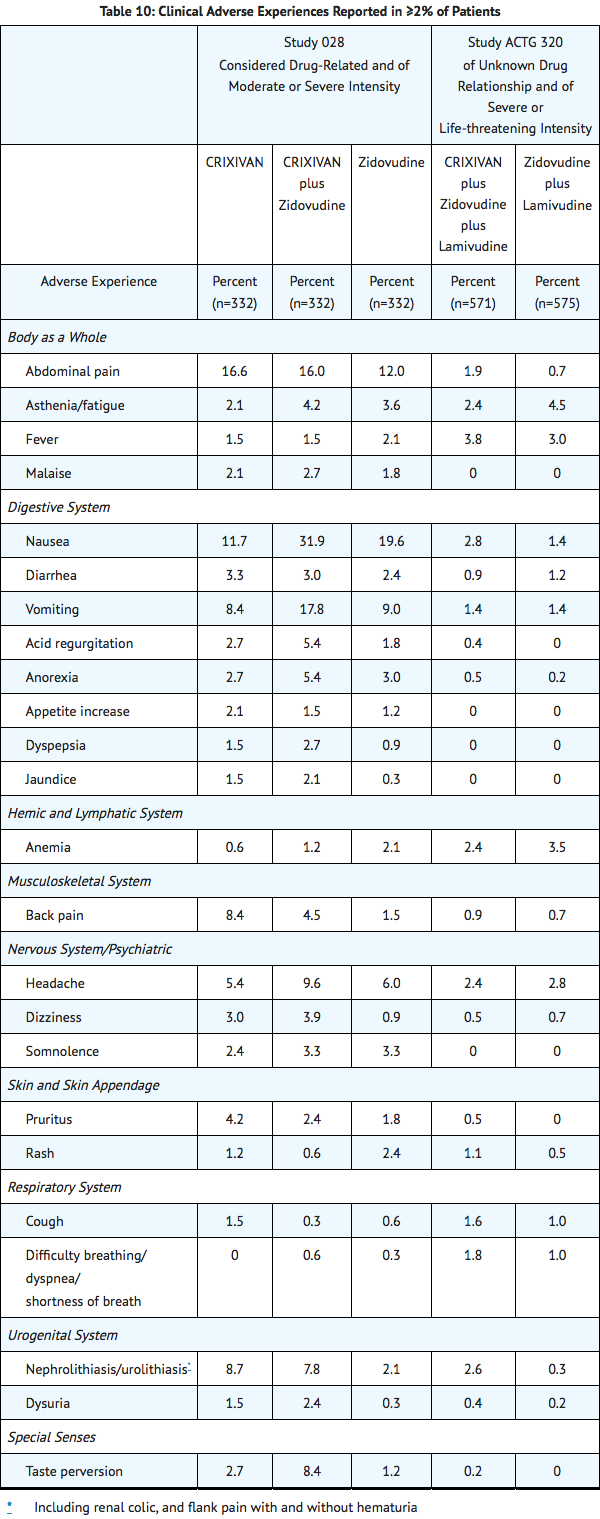
- In Phase I and II controlled trials, the following adverse events were reported significantly more frequently by those randomized to the arms containing Indinavir than by those randomized to nucleoside analogues: rash, upper respiratory infection, dry skin, pharyngitis, taste perversion.
Selected laboratory abnormalities of severe or life-threatening intensity reported in patients treated with Indinavir alone, Indinavir in combination with zidovudine or zidovudine plus lamivudine, zidovudine alone, or zidovudine plus lamivudine are presented in Table 11
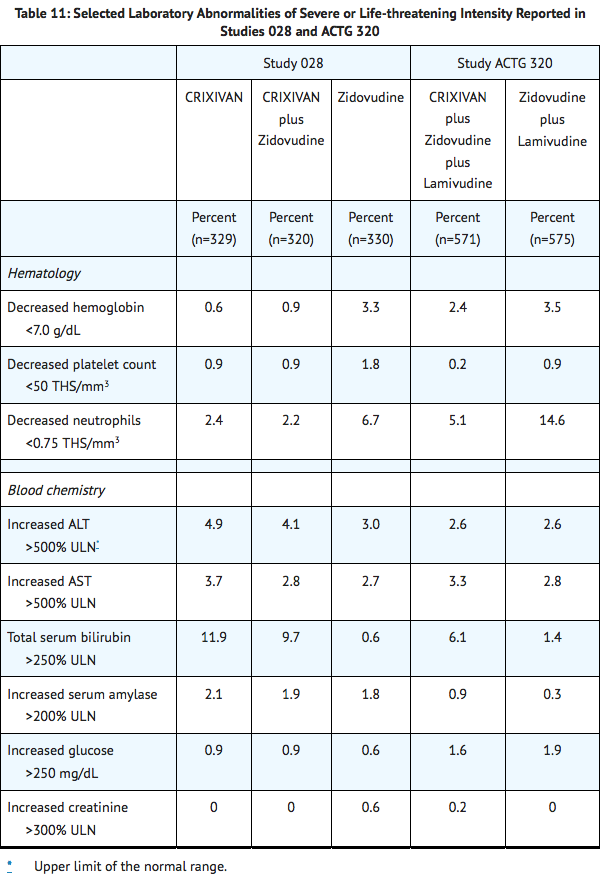
|postmarketing=*Body As A Whole: redistribution/accumulation of body fat.
- Cardiovascular System: cardiovascular disorders including myocardial infarction and angina pectoris; cerebrovascular disorder.
- Digestive System: liver function abnormalities; hepatitis including reports of hepatic failure; pancreatitis; jaundice; abdominal distention; dyspepsia.
- Hematologic: increased spontaneous bleeding in patients with hemophilia; acute hemolytic anemia.
- Endocrine/Metabolic: new onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, hyperglycemia.
- Hypersensitivity: anaphylactoid reactions; urticaria; vasculitis.
- Musculoskeletal System: arthralgia, periarthritis.
- Nervous System/Psychiatric: oral paresthesia; depression.
- Skin and Skin Appendage: rash including erythema multiforme and Stevens-Johnson syndrome; hyperpigmentation; alopecia; ingrown toenails and/or paronychia; pruritus.
- Urogenital System: nephrolithiasis/urolithiasis, in some cases resulting in renal insufficiency or acute renal failure, pyelonephritis with or without bacteremia; interstitial nephritis sometimes with indinavir crystal deposits; in some patients, the interstitial nephritis did not resolve following discontinuation of Indinavir; renal insufficiency; renal failure; leukocyturia, crystalluria; dysuria.
- Laboratory Abnormalities: Increased serum triglycerides; increased serum cholesterol.
|drugInteractions=*Indinavir is an inhibitor of the cytochrome P450 isoform CYP3A4. Coadministration of Indinavir and drugs primarily metabolized by CYP3A4 may result in increased plasma concentrations of the other drug, which could increase or prolong its therapeutic and adverse effects.
- Indinavir is metabolized by CYP3A4. Drugs that induce CYP3A4 activity would be expected to increase the clearance of indinavir, resulting in lowered plasma concentrations of indinavir. Coadministration of Indinavir and other drugs that inhibit CYP3A4 may decrease the clearance of indinavir and may result in increased plasma concentrations of indinavir.
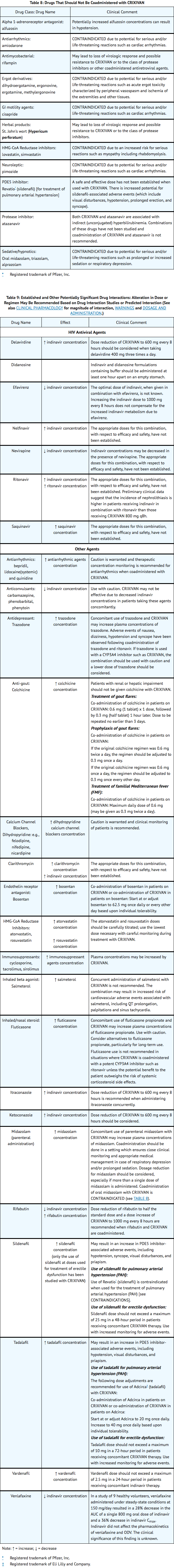
|FDAPregCat=C |useInPregnancyFDA=*Developmental toxicity studies were performed in rabbits (at doses up to 240 mg/kg/day), dogs (at doses up to 80 mg/kg/day), and rats (at doses up to 640 mg/kg/day). The highest doses in these studies produced systemic exposures in these species comparable to or slightly greater than human exposure. No treatment-related external, visceral, or skeletal changes were observed in rabbits or dogs. No treatment-related external or visceral changes were observed in rats. Treatment-related increases over controls in the incidence of supernumerary ribs (at exposures at or below those in humans) and of cervical ribs (at exposures comparable to or slightly greater than those in humans) were seen in rats. In all three species, no treatment-related effects on embryonic/fetal survival or fetal weights were observed.
- In rabbits, at a maternal dose of 240 mg/kg/day, no drug was detected in fetal plasma 1 hour after dosing. Fetal plasma drug levels 2 hours after dosing were approximately 3% of maternal plasma drug levels. In dogs, at a maternal dose of 80 mg/kg/day, fetal plasma drug levels were approximately 50% of maternal plasma drug levels both 1 and 2 hours after dosing. In rats, at maternal doses of 40 and 640 mg/kg/day, fetal plasma drug levels were approximately 10 to 15% and 10 to 20% of maternal plasma drug levels 1 and 2 hours after dosing, respectively.
- Indinavir was administered to Rhesus monkeys during the third trimester of pregnancy (at doses up to 160 mg/kg twice daily) and to neonatal Rhesus monkeys (at doses up to 160 mg/kg twice daily). When administered to neonates, indinavir caused an exacerbation of the transient physiologic hyperbilirubinemia seen in this species after birth; serum bilirubin values were approximately fourfold above controls at 160 mg/kg twice daily. A similar exacerbation did not occur in neonates after in utero exposure to indinavir during the third trimester of pregnancy. In Rhesus monkeys, fetal plasma drug levels were approximately 1 to 2% of maternal plasma drug levels approximately 1 hour after maternal dosing at 40, 80, or 160 mg/kg twice daily.
- Hyperbilirubinemia has occurred during treatment with Indinavir It is unknown whether Indinavir administered to the mother in the perinatal period will exacerbate physiologic hyperbilirubinemia in neonates.
- There are no adequate and well-controlled studies in pregnant patients. Indinavir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- A Indinavir dose of 800 mg every 8 hours (with zidovudine 200 mg every 8 hours and lamivudine 150 mg twice a day) has been studied in 16 HIV-infected pregnant patients at 14 to 28 weeks of gestation at enrollment (study PACTG 358). Given the substantially lower antepartum exposures observed and the limited data in this patient population, indinavir use is not recommended in HIV-infected pregnant patients
|useInNursing=*Studies in lactating rats have demonstrated that indinavir is excreted in milk. Although it is not known whether Indinavir is excreted in human milk, there exists the potential for adverse effects from indinavir in nursing infants. Mothers should be instructed to discontinue nursing if they are receiving Indinavir This is consistent with the recommendation by the U.S. Public Health Service Centers for Disease Control and Prevention that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. |useInPed=*The optimal dosing regimen for use of indinavir in pediatric patients has not been established. A dose of 500 mg/m2 every eight hours has been studied in uncontrolled studies of 70 children, 3 to 18 years of age. The pharmacokinetic profiles of indinavir at this dose were not comparable to profiles previously observed in adults receiving the recommended dose (see CLINICAL PHARMACOLOGY, PEDIATRIC). Although viral suppression was observed in some of the 32 children who were followed on this regimen through 24 weeks, a substantially higher rate of nephrolithiasis was reported when compared to adult historical data (see WARNINGS, NEPHROLITHIASIS/UROLITHIASIS). Physicians considering the use of indinavir in pediatric patients without other protease inhibitor options should be aware of the limited data available in this population and the increased risk of nephrolithiasis. |useInGeri=*Clinical studies of Indinavir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. |useInGender=*The effect of gender on the pharmacokinetics of indinavir was evaluated in 10 HIV seropositive women who received Indinavir 800 mg every 8 hours with zidovudine 200 mg every 8 hours and lamivudine 150 mg twice a day for one week. Indinavir pharmacokinetic parameters in these women were compared to those in HIV seropositive men (pooled historical control data). Differences in indinavir exposure, peak concentrations, and trough concentrations between males and females are shown in TABLE 1 below:
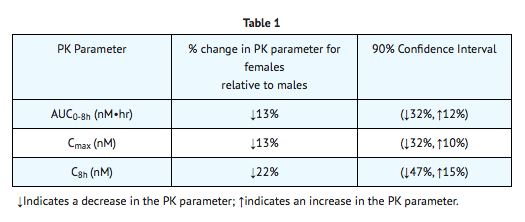
|useInRace=*Pharmacokinetics of indinavir appear to be comparable in Caucasians and Blacks based on pharmacokinetic studies including 42 Caucasians (26 HIV-positive) and 16 Blacks (4 HIV-positive). |useInRenalImpair=*The pharmacokinetics of indinavir have not been studied in patients with renal insufficiency. |useInHepaticImpair=*Patients with mild to moderate hepatic insufficiency and clinical evidence of cirrhosis had evidence of decreased metabolism of indinavir resulting in approximately 60% higher mean AUC following a single 400-mg dose (n=12). The half-life of indinavir increased to 2.8 ± 0.5 hours. Indinavir pharmacokinetics have not been studied in patients with severe hepatic insufficiency. |overdose=*There have been more than 60 reports of acute or chronic human overdosage (up to 23 times the recommended total daily dose of 2400 mg) with Indinavir The most commonly reported symptoms were renal (e.g., nephrolithiasis/urolithiasis, flank pain, hematuria) and gastrointestinal (e.g., nausea, vomiting, diarrhea). It is not known whether Indinavir is dialyzable by peritoneal or hemodialysis. |drugBox={{Drugbox2 | Verifiedfields = changed | verifiedrevid = 477168143 | IUPAC_name = (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]carbamoyl}butyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide | image = Indinavir Structure.png | width = 250 | image2 = Indinavir ball-and-stick.png
| tradename = Indinavir | Drugs.com = Monograph | MedlinePlus = a696028 | licence_US = Indinavir | pregnancy_US = C | legal_status = | routes_of_administration = Oral
| bioavailability = | protein_bound = 60% | metabolism = Hepatic via CYP3A4 | elimination_half-life = 1.8 (± 0.4) hours
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 150378-17-9
| ATC_prefix = J05
| ATC_suffix = AE02
| ATC_supplemental =
| PubChem = 5362440
| DrugBank_Ref =
| DrugBank = DB00224
| ChemSpiderID_Ref =
| ChemSpiderID = 4515036
| NIAID_ChemDB = 005824
| UNII_Ref =
| UNII = 9MG78X43ZT
| KEGG_Ref =
| KEGG = C07051
| ChEBI_Ref =
| ChEBI = 44032
| ChEMBL_Ref =
| ChEMBL = 540914
| PDB_ligand = MK1
| C=36 | H=47 | N=5 | O=4
| molecular_weight = 613.79 g/mol
| smiles = CC(C)(C)NC(=O)[C@@H]1CN(CCN1C[C@H](C[C@@H](Cc2ccccc2)C(=O)N[C@H]3c4ccccc4C[C@H]3O)O)Cc5cccnc5
| InChI = 1/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1
| InChIKey = CBVCZFGXHXORBI-PXQQMZJSBP
| StdInChI_Ref =
| StdInChI = 1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1
| StdInChIKey_Ref =
| StdInChIKey = CBVCZFGXHXORBI-PXQQMZJSSA-N
}}
|mechAction=*HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles.
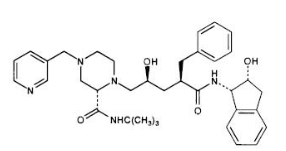
|structure=*The chemical name for indinavir sulfate is 1(1S,2R),5(S)-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-2-(1,1-dimethylethyl)amino carbonyl-4-(3-pyridinylmethyl)-1-piperazinyl-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula:
|PK=====Absorption====
- Indinavir was rapidly absorbed in the fasted state with a time to peak plasma concentration (Tmax) of 0.8 ± 0.3 hours (mean ± S.D.) (n=11). A greater than dose-proportional increase in indinavir plasma concentrations was observed over the 200-1000 mg dose range. At a dosing regimen of 800 mg every 8 hours, steady-state area under the plasma concentration time curve (AUC) was 30,691 ± 11,407 nM•hour (n=16), peak plasma concentration (Cmax) was 12,617 ± 4037 nM (n=16), and plasma concentration eight hours post dose (trough) was 251 ± 178 nM (n=16).
- Effect of Food on Oral Absorption: Administration of indinavir with a meal high in calories, fat, and protein (784 kcal, 48.6 g fat, 31.3 g protein) resulted in a 77% ± 8% reduction in AUC and an 84% ± 7% reduction in Cmax (n=10). Administration with lighter meals (e.g., a meal of dry toast with jelly, apple juice, and coffee with skim milk and sugar or a meal of corn flakes, skim milk and sugar) resulted in little or no change in AUC, Cmax or trough concentration.
Distribution
- Indinavir was approximately 60% bound to human plasma proteins over a concentration range of 81 nM to 16,300 nM.
Metabolism
- Following a 400-mg dose of 14C-indinavir, 83 ± 1% (n=4) and 19 ± 3% (n=6) of the total radioactivity was recovered in feces and urine, respectively; radioactivity due to parent drug in feces and urine was 19.1% and 9.4%, respectively. Seven metabolites have been identified, one glucuronide conjugate and six oxidative metabolites. In vitro studies indicate that cytochrome P-450 3A4 (CYP3A4) is the major enzyme responsible for formation of the oxidative metabolites.
Elimination
- Less than 20% of indinavir is excreted unchanged in the urine. Mean urinary excretion of unchanged drug was 10.4 ± 4.9% (n=10) and 12.0 ± 4.9% (n=10) following a single 700-mg and 1000-mg dose, respectively. Indinavir was rapidly eliminated with a half-life of 1.8 ± 0.4 hours (n=10). Significant accumulation was not observed after multiple dosing at 800 mg every 8 hours.
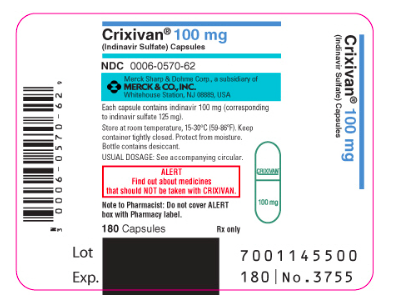
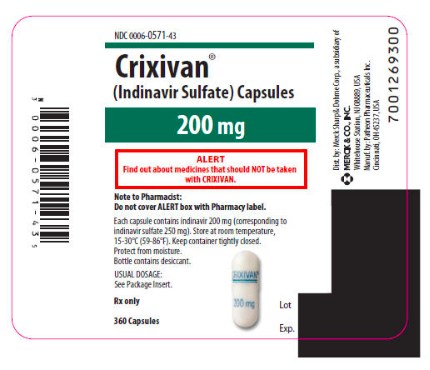
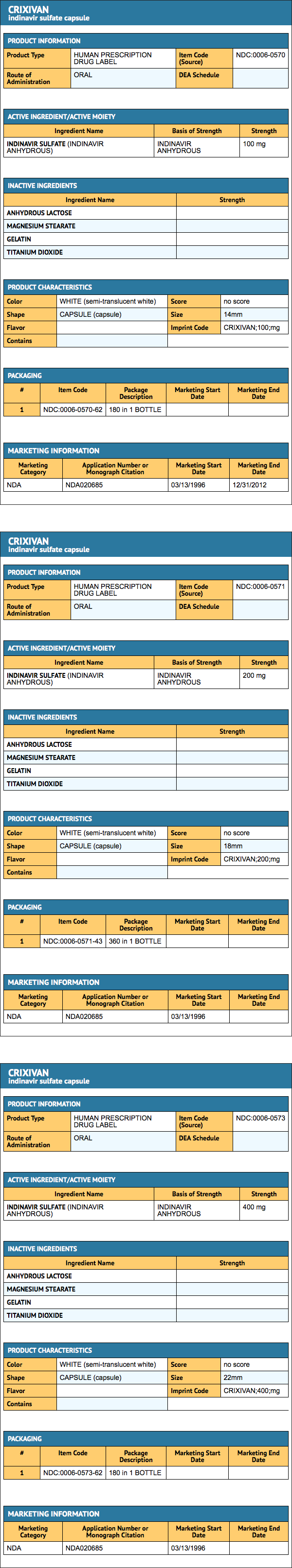
|howSupplied=Indinavur Capsules are supplied as follows:
- No. 3756 — 200 mg capsules: semi-translucent white capsules coded "Indinavur™ 200 mg" in blue. Available as:
- NDC 0006-0571-43 unit-of-use bottles of 360 (with desiccant).
- No. 3758 — 400 mg capsules: semi-translucent white capsules coded "Indinavur™ 400 mg" in green. Available as:
- NDC 0006-0573-62 unit-of-use bottles of 180 (with desiccant)
|storage=*Bottles: Store in a tightly-closed container at room temperature, 15-30°C (59-86°F). Protect from moisture.
- Indinavur Capsules are sensitive to moisture. Indinavur should be dispensed and stored in the original container. The desiccant should remain in the original bottle.
|packLabel=
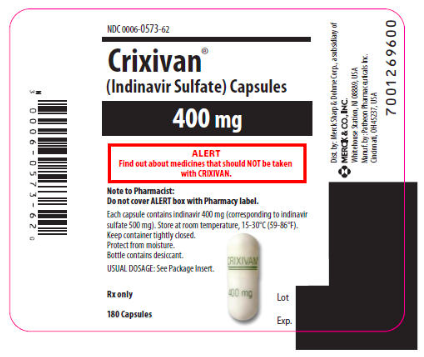
|alcohol=*Alcohol-Indinavir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=*Crixivan }}
- ↑ "Imprimir Management Of Adults And Children With Nonoccupational Exposure To HIV". line feed character in
|title=at position 9 (help) - ↑ "Imprimir Management Of Adults And Children With Nonoccupational Exposure To HIV". line feed character in
|title=at position 9 (help)