Human papillomavirus pathophysiology
|
Human papillomavirus Microchapters |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Human papillomavirus pathophysiology On the Web |
|
American Roentgen Ray Society Images of Human papillomavirus pathophysiology |
|
Risk calculators and risk factors for Human papillomavirus pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Aysha Anwar, M.B.B.S[2],Seyedmahdi Pahlavani, M.D. [3]
Overview
Human papilloma virus is usually transmitted via the sexual route to the human host.[1] HPV life cycle is linked to epithelial differentiation and maturation of host keratinocytes, with transcription of specific gene products at every level.[2][3] The pathogenesis of HPV infection causing cancer is mainly linked to high risk types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). E6 and E7 protein products of HPV interact with two important cell cycle regulatory protiens, P53 and Rb proteins of host cell, causing unchecked cellular replication accumulating mutations leading to cancer.[4][5][6]
Pathophysiology
Transmission
- Human papilloma virus is usually transmitted via the sexual route to the human host.[1]
- Different types of HPV has a predilection for different types of epithelial tissue.[7]
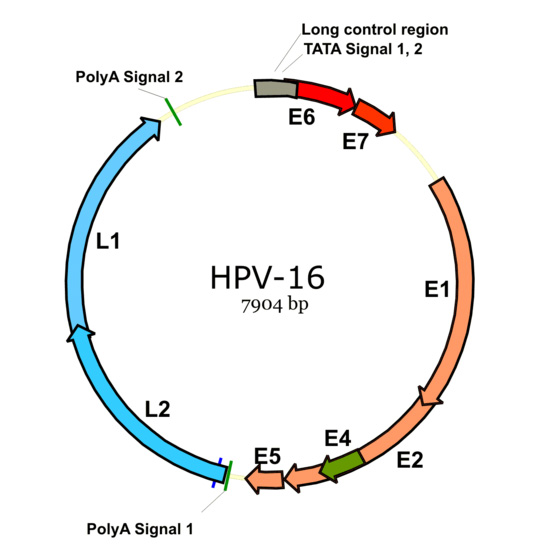
HPV life cycle
- HPV life cycle is linked to epithelial differentiation and maturation of host keratinocytes, with transcription of specific gene products at every level.[2][3][8]
- HPV primarily infects basal cell layer of stratified squamous keratinised epithelium.[9]
- Following transmission, the HPV uses the microabrasions to enter the basal stem cells via tissue specific heparan sulfate proteoglycans through clathrin-mediated endocytosis and/or caveolin-mediated endocytosis depending on the type of HPV.[10]
- It than undergoes viral uncoating and viral DNA genome is than transported to nucleus maintaining a low copy number 10-200 viral genomes per cell (episome form).[11]
- A sophisticated transcriptional cascade then occurs as the host keratinocyte begins to divide and become increasingly differentiated in the upper layers of the epithelium.
- HPV uses host DNA replicative machinery to multiply as it lacks DNA polymerase activity.
- Specific viral genes are transcribed at every level of keratinocyte differention.
- Early proteins: E1, E2, E3, E4, E5, E6 and E7 proteins are synthesized primarily in middle layers, for reactivation of replication process in the differentiated cells.
- Late proteins: L1, L2 proteins are transcribed in the most superficial layers for virion assesmbly, release and reinfection, as they code for capsid proteins.[12]
Pathogenesis of HPV induced cancers
The pathogenesis of HPV infection causing cancer is mainly linked to high risk types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). Following HPV proteins play a significant role in the development of cancers associated with HPV.[13]
E6 and E7 proteins
E6 and E7 protein products of HPV interact with two important cell cycle regulatory protiens, P53 and Rb proteins of host cell, causing unchecked cellular replication accumulating mutations leading to cancer.[4]
- Inhibition of P53
- P53 protein is a cellular check point at G0/G1 to S phase of cell cycle and is also responsible for cell apoptosis for unrepaired DNA mutations. E6 protein binds P53 which results in degradation of P53, leaving cell without any check for mutations and unregulated cell.[5][14]
- growth.[15][16][17][18][19]
- Inhibition of Rb protein
- Rb protein is negative regulator of cell growth. It binds E2F transcription factor which controls DNA replication and cyclin protein induced entering of cell into S phase of cell cycle. E7 protein binds Rb/E2F, releasing E2F from the inhibitory effect of Rb causing increased cyclin induced entry of cell into S phase of cell cycle, resulting in increased replication rate of cells accumulating mutations.[19][20][6][21]
HPV-Induced Diseases
| Disease | HPV type |
|---|---|
| Common warts | 2, 7 |
| Plantar warts | 1, 2, 4 |
| Flat cutaneous warts | 3, 10 |
| Anogenital warts | 6, 11, 42, 43, 44, 55 and others |
| Genital malignancies | 16, 18, 31, 33, 35, 39, 45, 51 |
| Epidermodysplasia verruciformis | more than 15 types |
| Focal epithelial hyperplasia (oral) | 13, 32 |
| Oral papillomas | 6, 7, 11, 16, 32 |
References
- ↑ 1.0 1.1 Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffie K, Shvetsov YB; et al. (2008). "Transmission of human papillomavirus in heterosexual couples". Emerg Infect Dis. 14 (6): 888–94. doi:10.3201/eid1406.070616. PMC 2600292. PMID 18507898.
- ↑ 2.0 2.1 Doorbar J (2005). "The papillomavirus life cycle". J Clin Virol. 32 Suppl 1: S7–15. doi:10.1016/j.jcv.2004.12.006. PMID 15753007.
- ↑ 3.0 3.1 Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR; et al. (2012). "The biology and life-cycle of human papillomaviruses". Vaccine. 30 Suppl 5: F55–70. doi:10.1016/j.vaccine.2012.06.083. PMID 23199966.
- ↑ 4.0 4.1 Moody CA, Laimins LA (2010). "Human papillomavirus oncoproteins: pathways to transformation". Nat Rev Cancer. 10 (8): 550–60. doi:10.1038/nrc2886. PMID 20592731.
- ↑ 5.0 5.1 Masuda H, Miller C, Koeffler HP, Battifora H, Cline MJ (1987). "Rearrangement of the p53 gene in human osteogenic sarcomas". Proc Natl Acad Sci U S A. 84 (21): 7716–9. PMC 299371. PMID 2823272.
- ↑ 6.0 6.1 Tommasino M, Adamczewski JP, Carlotti F, Barth CF, Manetti R, Contorni M; et al. (1993). "HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A." Oncogene. 8 (1): 195–202. PMID 8380917.
- ↑ Rintala MA, Grénman SE, Puranen MH, Isolauri E, Ekblad U, Kero PO; et al. (2005). "Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland". J Clin Microbiol. 43 (1): 376–81. doi:10.1128/JCM.43.1.376-381.2005. PMC 540188. PMID 15634997.
- ↑ Doorbar J (2007). "Papillomavirus life cycle organization and biomarker selection". Dis Markers. 23 (4): 297–313. PMC 3851388. PMID 17627064.
- ↑ Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM (2009). "The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding". Proc Natl Acad Sci U S A. 106 (48): 20458–63. doi:10.1073/pnas.0908502106. PMC 2787115. PMID 19920181.
- ↑ Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM (2009). "Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus". J Virol. 83 (5): 2067–74. doi:10.1128/JVI.02190-08. PMC 2643729. PMID 19073722.
- ↑ Bedell MA, Hudson JB, Golub TR, Turyk ME, Hosken M, Wilbanks GD; et al. (1991). "Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation". J Virol. 65 (5): 2254–60. PMC 240574. PMID 1850010.
- ↑ Yang R, Yutzy WH, Viscidi RP, Roden RB (2003). "Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection". J Biol Chem. 278 (14): 12546–53. doi:10.1074/jbc.M208691200. PMID 12560332.
- ↑ de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B; et al. (2010). "Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study". Lancet Oncol. 11 (11): 1048–56. doi:10.1016/S1470-2045(10)70230-8. PMID 20952254.
- ↑ Hinds P, Finlay C, Levine AJ (1989). "Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation". J Virol. 63 (2): 739–46. PMC 247745. PMID 2642977.
- ↑ Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (1993). "The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53". Cell. 75 (3): 495–505. PMID 8221889.
- ↑ Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM (1995). "p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells". Cancer Res. 55 (19): 4420–4. PMID 7671255.
- ↑ Werness BA, Levine AJ, Howley PM (1990). "Association of human papillomavirus types 16 and 18 E6 proteins with p53". Science. 248 (4951): 76–9. PMID 2157286.
- ↑ Magal SS, Jackman A, Pei XF, Schlegel R, Sherman L (1998). "Induction of apoptosis in human keratinocytes containing mutated p53 alleles and its inhibition by both the E6 and E7 oncoproteins". Int J Cancer. 75 (1): 96–104. PMID 9426696.
- ↑ 19.0 19.1 Demers GW, Foster SA, Halbert CL, Galloway DA (1994). "Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7". Proc Natl Acad Sci U S A. 91 (10): 4382–6. PMC 43789. PMID 8183918.
- ↑ Pagano M, Dürst M, Joswig S, Draetta G, Jansen-Dürr P (1992). "Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells". Oncogene. 7 (9): 1681–6. PMID 1323816.
- ↑ Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ; et al. (1999). "The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth". EMBO J. 18 (9): 2449–58. doi:10.1093/emboj/18.9.2449. PMC 1171327. PMID 10228159.