Hepatitis A laboratory findings: Difference between revisions
Joao Silva (talk | contribs) No edit summary |
Joao Silva (talk | contribs) No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Hepatitis A cannot be differentiated from other types of viral hepatitis on the basis of clinical or epidemiologic features alone. Serologic testing to detect immunoglobulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection. In most persons, IgM anti-HAV becomes detectable 5-10 days before the onset of symptoms and can persist for up to 6 months after infection.<ref>Bower WA, Nainan OV, Margolis HS. Duration of viremia in naturally-acquired hepatitis A viral infections. [Abstract 103] In: Abstracts of the Infectious Diseases Society of America 35th Annual Meeting. Alexandria, VA: Infectious Diseases Society of America, 1997.</ref><ref name="pmid3759243">{{cite journal |author=Liaw YF, Yang CY, Chu CM, Huang MJ |title=Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak |journal=[[Infection]] |volume=14 |issue=4 |pages=156–8 |year=1986 |pmid=3759243 |doi= |url= |accessdate=2012-02-28}}</ref> Immunoglobulin G (IgG) anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and confers lifelong protection against the disease. <ref name="pmid7876654">{{cite journal |author=Stapleton JT |title=Host immune response to hepatitis A virus |journal=[[The Journal of Infectious Diseases]] |volume=171 Suppl 1 |issue= |pages=S9–14 |year=1995 |month=March |pmid=7876654 |doi= |url=http://www.jid.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=7876654 |accessdate=2012-02-28}}</ref> Commercial diagnostic tests are available for the detection of IgM and total (IgM and IgG) anti-HAV in serum. | |||
==Laboratory Findings== | |||
Hepatitis A cannot be differentiated from other types of viral hepatitis on the basis of clinical or epidemiologic features alone. | |||
* Serologic testing to detect immunoglobulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection. | |||
* In most persons, IgM anti-HAV becomes detectable 5-10 days before the onset of symptoms and can persist for up to 6 months after infection.<ref>Bower WA, Nainan OV, Margolis HS. Duration of viremia in naturally-acquired hepatitis A viral infections. [Abstract 103] In: Abstracts of the Infectious Diseases Society of America 35th Annual Meeting. Alexandria, VA: Infectious Diseases Society of America, 1997.</ref><ref name="pmid3759243">{{cite journal |author=Liaw YF, Yang CY, Chu CM, Huang MJ |title=Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak |journal=[[Infection]] |volume=14 |issue=4 |pages=156–8 |year=1986 |pmid=3759243 |doi= |url= |accessdate=2012-02-28}}</ref> | |||
* Immunoglobulin G (IgG) anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and confers lifelong protection against the disease. <ref name="pmid7876654">{{cite journal |author=Stapleton JT |title=Host immune response to hepatitis A virus |journal=[[The Journal of Infectious Diseases]] |volume=171 Suppl 1 |issue= |pages=S9–14 |year=1995 |month=March |pmid=7876654 |doi= |url=http://www.jid.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=7876654 |accessdate=2012-02-28}}</ref> Commercial diagnostic tests are available for the detection of IgM and total (IgM and IgG) anti-HAV in serum. | |||
{{#widget:SchemaSnippet}} | {{#widget:SchemaSnippet}} | ||
== | * HAV RNA can be detected in the blood and stool of most persons during the acute phase of infection by using nucleic acid amplification methods, and nucleic acid sequencing has been used to determine the relatedness of HAV isolates. <ref name="pmid10029643">{{cite journal |author=Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, Goldstein ST, Gensheimer KF, Bell BP, Shapiro CN, Alter MJ, Margolis HS |title=A multistate, foodborne outbreak of hepatitis A. National Hepatitis A Investigation Team |journal=[[The New England Journal of Medicine]] |volume=340 |issue=8 |pages=595–602 |year=1999 |month=February |pmid=10029643 |doi=10.1056/NEJM199902253400802 |url=http://dx.doi.org/10.1056/NEJM199902253400802 |accessdate=2012-02-28}}</ref> However, these methods, available in only a limited number of research laboratories, generally are not used for diagnostic purposes. | ||
[[Image:HAV Infection.png|left|thumb|300px|Serum IgG, IgM and ALT following Hepatitis A virus infection]] | [[Image:HAV Infection.png|left|thumb|300px|Serum IgG, IgM and ALT following Hepatitis A virus infection]] | ||
HAV RNA can be detected in the blood and stool of the majority of persons during the acute phase of infection by using nucleic acid amplification methods, and nucleic acid sequencing has been used to determine the relatedness of HAV isolates for epidemiologic investigations. However, only a limited number of research laboratories have the capacity to use these methods. | * Serologic testing to detect immunoglobulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection. Sensitive tests for IgM and immunoglobulin G (IgG) anti-HAV in saliva have been developed but are not licensed in the United States. In the majority of persons, serum IgM anti-HAV becomes detectable 5--10 days before onset of symptoms. IgG anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and provides lifelong protection against the disease. Two serologic tests are licensed for the detection of antibodies to HAV: 1) IgM anti-HAV and 2) total anti-HAV (i.e., IgM and IgG anti-HAV, referred to in this report as anti-HAV). In the majority of patients, IgM anti-HAV declines to undetectable levels <6 months after infection. However, persons who test positive for IgM anti-HAV >1 year after infection have been reported, as have likely false-positive tests in persons without evidence of recent HAV infection. Total anti-HAV testing is used in epidemiologic studies to measure the prevalence of previous infection or by clinicians to determine whether a person with an indication for pre-exposure prophylaxis is already immune. | ||
* HAV RNA can be detected in the blood and stool of the majority of persons during the acute phase of infection by using nucleic acid amplification methods, and nucleic acid sequencing has been used to determine the relatedness of HAV isolates for epidemiologic investigations. However, only a limited number of research laboratories have the capacity to use these methods. | |||
===Liver Function Tests=== | ===Liver Function Tests=== | ||
Revision as of 14:02, 28 July 2014
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A laboratory findings On the Web |
|
American Roentgen Ray Society Images of Hepatitis A laboratory findings |
|
Risk calculators and risk factors for Hepatitis A laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
Laboratory Findings
Hepatitis A cannot be differentiated from other types of viral hepatitis on the basis of clinical or epidemiologic features alone.
- Serologic testing to detect immunoglobulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection.
- In most persons, IgM anti-HAV becomes detectable 5-10 days before the onset of symptoms and can persist for up to 6 months after infection.[1][2]
- Immunoglobulin G (IgG) anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and confers lifelong protection against the disease. [3] Commercial diagnostic tests are available for the detection of IgM and total (IgM and IgG) anti-HAV in serum.
- HAV RNA can be detected in the blood and stool of most persons during the acute phase of infection by using nucleic acid amplification methods, and nucleic acid sequencing has been used to determine the relatedness of HAV isolates. [4] However, these methods, available in only a limited number of research laboratories, generally are not used for diagnostic purposes.
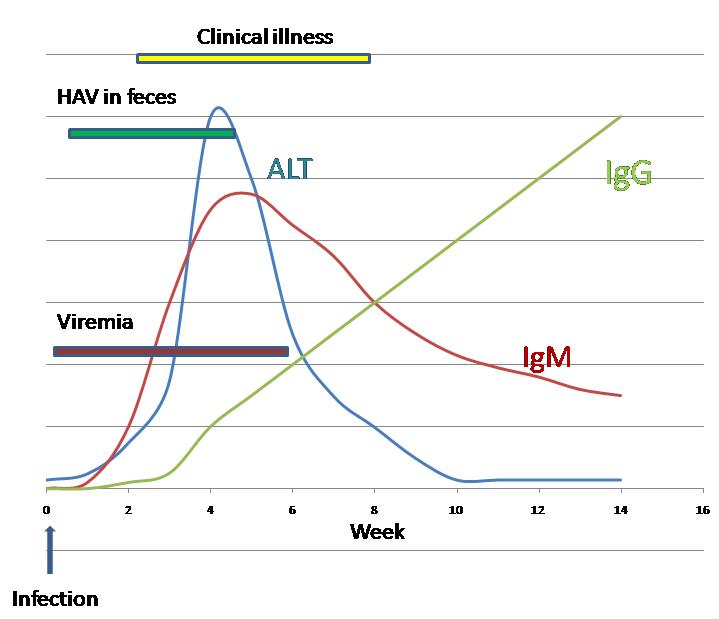
- Serologic testing to detect immunoglobulin M (IgM) antibody to the capsid proteins of HAV (IgM anti-HAV) is required to confirm a diagnosis of acute HAV infection. Sensitive tests for IgM and immunoglobulin G (IgG) anti-HAV in saliva have been developed but are not licensed in the United States. In the majority of persons, serum IgM anti-HAV becomes detectable 5--10 days before onset of symptoms. IgG anti-HAV, which appears early in the course of infection, remains detectable for the person's lifetime and provides lifelong protection against the disease. Two serologic tests are licensed for the detection of antibodies to HAV: 1) IgM anti-HAV and 2) total anti-HAV (i.e., IgM and IgG anti-HAV, referred to in this report as anti-HAV). In the majority of patients, IgM anti-HAV declines to undetectable levels <6 months after infection. However, persons who test positive for IgM anti-HAV >1 year after infection have been reported, as have likely false-positive tests in persons without evidence of recent HAV infection. Total anti-HAV testing is used in epidemiologic studies to measure the prevalence of previous infection or by clinicians to determine whether a person with an indication for pre-exposure prophylaxis is already immune.
- HAV RNA can be detected in the blood and stool of the majority of persons during the acute phase of infection by using nucleic acid amplification methods, and nucleic acid sequencing has been used to determine the relatedness of HAV isolates for epidemiologic investigations. However, only a limited number of research laboratories have the capacity to use these methods.
Liver Function Tests
Liver enzymes such as aminotransferase and alkaline phosphatase are elevated with high levels of serum total and direct bilirubin among patients with Hepatitis A.[5] The serum levels of alanine aminotransferase (ALT) are usually higher than aspartate aminotransferase (AST). Bilirubin levels tends to increase after the elevation of serum aminotransferase.
Other Laboratory Findings
- Elevated erythrocyte sedimentation rate (ESR)
- Acute phase reactants
- Immunoglobulins are the other lab findings that may be evident in patients infected with Hepatitis A.
Liver Biopsy
The role of a liver biopsy is minimal in the diagnosis of hepatitis A. It may be used in cases involving chronic relapsing hepatitis A or when the diagnosis is unclear.
References
- ↑ Bower WA, Nainan OV, Margolis HS. Duration of viremia in naturally-acquired hepatitis A viral infections. [Abstract 103] In: Abstracts of the Infectious Diseases Society of America 35th Annual Meeting. Alexandria, VA: Infectious Diseases Society of America, 1997.
- ↑ Liaw YF, Yang CY, Chu CM, Huang MJ (1986). "Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak". Infection. 14 (4): 156–8. PMID 3759243.
|access-date=requires|url=(help) - ↑ Stapleton JT (1995). "Host immune response to hepatitis A virus". The Journal of Infectious Diseases. 171 Suppl 1: S9–14. PMID 7876654. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, Goldstein ST, Gensheimer KF, Bell BP, Shapiro CN, Alter MJ, Margolis HS (1999). "A multistate, foodborne outbreak of hepatitis A. National Hepatitis A Investigation Team". The New England Journal of Medicine. 340 (8): 595–602. doi:10.1056/NEJM199902253400802. PMID 10029643. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ Tong MJ, el-Farra NS, Grew MI (1995). "Clinical manifestations of hepatitis A: recent experience in a community teaching hospital". The Journal of Infectious Diseases. 171 Suppl 1: S15–8. PMID 7876641. Retrieved 2012-03-08. Unknown parameter
|month=ignored (help)