Goodpasture syndrome pathophysiology
| Title |
| https://https://www.youtube.com/watch?v=vAX2UMA8Oek%7C350}} |
|
Goodpasture syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Goodpasture syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Goodpasture syndrome pathophysiology |
|
Risk calculators and risk factors for Goodpasture syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Ali Poyan Mehr, M.D. [2]Associate Editor(s)-in-Chief: Krzysztof Wierzbicki M.D. [3]
Overview
- The overview section should include the disease name in the first sentence.
- The goal is to summarize the pathophysiology page in several sentences. This section can be the same as the pathophysiology segment on the overview page.
- To see an example of an overview section on a symptoms page, click here.
Template
- The overview is highly dependent on the individual disease pathophysiology. There is no specific template preference for the first sentence.
Template Sentences:
- Template Sentence 1: [Pathogen name] is usually transmitted via the [transmission route] route to the human host.
- Template Sentence 2: Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
- Template Sentence 3: On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
- Template Sentence 4: On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
- Template Sentence 5: [Disease name] is transmitted in [mode of genetic transmission] pattern.
- Template Sentence 6: [Disease/malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
- Template Sentence 7: Development of [disease name] is the result from multiple genetic mutations.
- Template Sentence 8: Genes involved in the pathogenesis of [disease name] include [gene1], [gene2], and [gene3].
- Template Sentence 9: The progression to [disease name] usually involves the [molecular pathway].
- Template Sentence 10: The pathophysiology of [disease name] depends on the histological subtype.
Examples:
- Example 1: Spores of C. difficile are transmitted via the fecal-oral route to the human host.
- Example 2: Following ingestion, the acid-resistant spores of C. difficile are able to survive the human gastric acidity.
- Example 3: Following ingestion, Shigella spp. uses the M cells of the GI tract to invade the epithelial cells of the large intestine.
- Example 4: Following transcytosis and macrophage apoptosis, Shigella avoids extracellular exposure and spreads intercellularly using actin polymerization processes (rocket propulsion).
- Example 5: On gross pathology, hyperemia with development of ulcers and edema are characteristic findings of shigellosis.
- Example 6: On microscopic histopathological analysis, infiltration of PMN and inflammatory pseudomembrane formation are characteristic findings of shigellosis.
- Example 7: Duchenne muscular dystrophy is transmitted in an X-linked recessive pattern.
- Example 8: Malignant melanoma arises from the epidermal melanocytes, which are neural crest cells normally involved in the synthesis of melanin (a brown pigment with photoprotective properties).
- Example 9: Development of melanoma is the result of multiple genetic mutations.
- Example 10: Genes involved in the pathogenesis of melanoma include p53, RB, ARF, and BRAF.
- Example 11: The progression to melanoma usually involves the serine-threonine kinases of the MAPK/ERK pathway (mitogen-activated protein kinase) following mutation of either the N-RAS or BRAF oncogene.
- Example 12: The pathophysiology of gallbladder cancer depends on the histological subtype.
Overview
The pathogenesis of Goodpasture syndrome is the presence of autoantibodies against the glomerular or alveolar basement membranes. The target antigen that has the strongest pathogenic effect on anti-GBM disease is the non-collagenous 1 domain of alpha-3 type IV collagen.[1] There is strong correlation of anti-glomerular basement membrane disease with allele HLA DRB1-1501.This allele is associated in causing renal injury. [2]
Pathogenesis
The pathogenesis of Goodpasture syndrome is the presence of autoantibodies against the glomerular or alveolar basement membranes. The target antigen that has the strongest pathogenic effect on anti-GBM disease is the non-collagenous 1 domain of alpha-3 type IV collagen.[1] There are 2 dominant epitopes, they are epitope A and epitope B. These epitopes are seen in the alpha-3 non-collagenous 1 domain of type IV collagen and antibodies do not normally bind unless a change has occurred in the non-cross linked hexamers or trimers. In Goodpasture syndrome these epitopes undergo a transitional change in the non-cross linked hexamers or trimers. This allows antibodies to be bound to the epitopes. The pathogenic role of epitopes such as epitope A, has been shown to affect the function of the renals, especially of the alpha-3 type IV collagen of non-collagenous 1 domain. Epitope B however is not able to induce Goodpasture syndrome by itself.[3] The cause of renal injury is due to anti-glomerular basement membrane antibodies ability to bind and activate complements and proteases, resulting in a interruption between the filtration barrier and the Bowman’s capsule. The interruption of the Bowman's capsule and filtration barrier, induces crescent formation of the renal, invokes proteinuria and induces T cells (CD4+ and CD8+), macrophages, and neutrophils.[4]
Factors that are associated with these process are due to oxidation, nitrosylation, glycation, increases in body temperature, or proteolytic cleavage. For example, it has been shown that proteolytic cleavage of disulfide bonds in non-cross linked hexamers has shown greater affinity for Goodpasture syndrome antibodies to bind. Environmental factors are also said to attribute to the disease such as exposures to hydrocarbons, exposure to formaldehyde or smoking tobacco. Smoking tobacco for instance causes sulfilimine bond substances to be inhibited. Inhibition of the sulfilimine bond substances cause non-cross linked hexamers to form.[5]
Genetics
There is strong correlation of anti-glomerular basement membrane disease with allele HLA DRB1-1501. This allele is associated in causing renal injury. [2] This allele is present in 80% of patients with Goodpasture syndrome.[6]
Associated Conditions
Microscopic Pathology
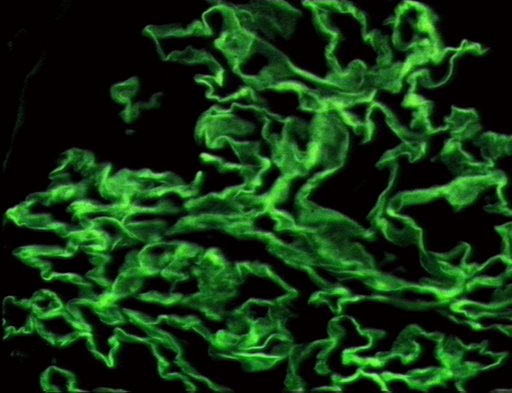
On microscopic histopathological analysis, early focal proliferative changes that display necrosis and crescent formation with an inflamed interstitial are seen. Under direct immunofluorescence, linear immunoglobulin G deposits are found encompassing the glomerular basement membrane and at times the distal tubular portion of the basement membrane.[7] The following are images of the microscopic pathology of crescent glomerulonephritis and the immunofluorescence of linear IgG. [8]
Pathophysiology
As with many autoimmune conditions, the precise cause of Goodpasture’s Syndrome is not yet known. It is believed to be a type II hypersensitivity reaction to Goodpasture’s antigens on the cells of the glomeruli of the kidneys and the pulmonary alveoli, specifically the basement membrane's (including a-3 chain of type IV collagen), whereby the immune system wrongly recognizes these cells as foreign and attacks and destroys them, as it would an invading pathogen.
Pathology
References
- ↑ 1.0 1.1 Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH (2009). "Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity". Kidney Int. 76 (10): 1108–15. doi:10.1038/ki.2009.348. PMID 19741587.
- ↑ 2.0 2.1 Xie LJ, Cui Z, Jia XY, Chen Z, Liu XR, Zhao MH (2015). "Coexistence of Anti-Glomerular Basement Membrane Antibodies and Anti-Neutrophil Cytoplasmic Antibodies in a Child With Human Leukocyte Antigen Susceptibility and Detailed Antibody Description: A Case Report". Medicine (Baltimore). 94 (29): e1179. doi:10.1097/MD.0000000000001179. PMC 4603008. PMID 26200622.
- ↑ Chen JL, Hu SY, Jia XY, Zhao J, Yang R, Cui Z; et al. (2013). "Association of epitope spreading of antiglomerular basement membrane antibodies and kidney injury". Clin J Am Soc Nephrol. 8 (1): 51–8. doi:10.2215/CJN.05140512. PMC 3531658. PMID 23085731.
- ↑ Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (2003). "Alport's syndrome, Goodpasture's syndrome, and type IV collagen". N Engl J Med. 348 (25): 2543–56. doi:10.1056/NEJMra022296. PMID 12815141.
- ↑ Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR; et al. (2010). "Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis". N Engl J Med. 363 (4): 343–54. doi:10.1056/NEJMoa0910500. PMC 4144421. PMID 20660402.
- ↑ Couser WG (2016). "Pathogenesis and treatment of glomerulonephritis-an update". J Bras Nefrol. 38 (1): 107–22. doi:10.5935/0101-2800.20160016. PMID 27049372.
- ↑ Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Pagliuca G; et al. (2015). "Goodpasture's syndrome: a clinical update". Autoimmun Rev. 14 (3): 246–53. doi:10.1016/j.autrev.2014.11.006. PMID 25462583.
- ↑ University of Pittsburgh Medical Center Pathology. www.path.upmc.edu/cases/case541.html Accessed on Novermber 2nd 2016
- ↑ http://picasaweb.google.com/mcmumbi/USMLEIIImages

