Ganciclovir description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
The Vitrasert Implant contains the antiviral drug ganciclovir. Each Vitrasert Implant contains a minimum of 4.5 mg ganciclovir.
Each Vitrasert Implant contains a ganciclovir tablet which contains the inactive ingredient, magnesium stearate (0.25%). Each tablet is coated with polyvinyl alcohol and ethylene vinyl acetate polymers.
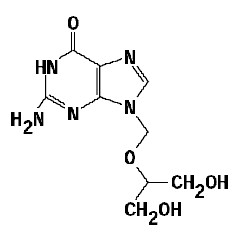
The chemical name of ganciclovir is 9-[[2-hydroxy -1- (hydroxymethyl)ethoxy] methyl]guanine, and has the following structure:
 |
References
- ↑ "http://www.drugbank.ca/system/fda_labels/DB01004.pdf?1265922812" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.