Fosfomycin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fosfomycin is an anti-bacterial agent that is FDA approved for the treatment of uncomplicated urinary tract infection. Common adverse reactions include diarrhea, vaginitis, nausea, headache, dizziness, asthenia, dyspepsia and elevation of eosinophil and WBC counts, bilirubin, ALT, AST, alkaline phosphatase and decrease in hematocrit, hemoglobin and platelet count.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Uncomplicated urinary tract infections
- Fosfomycin is indicated only for the treatment of uncomplicated urinary tract infections (acute cystitis) in women due to susceptible strains of Escherichia coli and Enterococcus faecalis.Fosfomycin is not indicated for the treatment of pyelonephritis or perinephric abscess.
- If persistence or reappearance of bacteriuria occurs after treatment with Fosfomycin, other therapeutic agents should be selected.
Dosing Information
- The recommended dosage for women 18 years of age and older for uncomplicated urinary tract infection (acute cystitis) is one sachet of Fosfomycin. Fosfomycin may be taken with or without food.
- Fosfomycin should not be taken in its dry form. Always mix Fosfomycin with water before ingesting.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fosfomycin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fosfomycin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in children age 12 years and under have not been established in adequate and well-controlled studies..
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Safety and effectiveness in children age 12 years and under have not been established in adequate and well-controlled studies..
Non–Guideline-Supported Use
Safety and effectiveness in children age 12 years and under have not been established in adequate and well-controlled studies..
Contraindications
- Fosfomycin is contraindicated in patients with known hypersensitivity to the drug.
Warnings
- Clostridium difficileassociated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Fosfomycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains ofC. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed againstC. difficilemay need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
- General
- Do not use more than one single dose of Fosfomycin to treat a single episode of acute cystitis. :*Repeated daily doses of Fosfomycin did not improve the clinical success or microbiological eradication rates compared to single dose therapy, but did increase the incidence of adverse events. Urine specimens for culture and susceptibility testing should be obtained before and after completion of therapy.
Adverse Reactions
Clinical Trials Experience
- In clinical studies, drug related adverse events which were reported in greater than 1% of the fosfomycin-treated study population are listed below:

- In clinical trials, the most frequently reported adverse events occurring in > 1 % of the study population regardless of drug relationship were:
- The following adverse events occurred in clinical trials at a rate of less than 1%, regardless of drug relationship:
- Abnormal stools, anorexia, constipation, dry mouth,dysuria, ear disorder, fever,flatulence, flu syndrome, hematuria, infection, insomnia, lymphadenopathy, menstrual disorder, migraine,myalgia, nervousness, paresthesia,pruritus, SGPT increased, skin disorder, somnolence, and vomiting.
- One patient developed unilateral optic neuritis, an event considered possibly related to Fosfomycin therapy.
Postmarketing Experience
- Serious adverse events from the marketing experience with Fosfomycin outside of the United States have been rarely reported and include: angioedema, aplasticanemia, asthma (exacerbation),cholestatic jaundice, hepatic necrosis, and toxic megacolon.
- Although causality has not been established, during post marketing surveillance, the following events have occurred in patients prescribed Fosfomycin: anaphylaxis and hearing loss.
Drug Interactions
- Metoclopramide
- When coadministered with Fosfomycin,metoclopramide, a drug which increases gastrointestinal motility, lowers the serum concentration and urinary excretion of fosfomycin. Other drugs that increase gastrointestinal motility may produce similar effects.
- Cimetidine
- Cimetidine does not affect the pharmacokinetics of fosfomycin when coadministered with Fosfomycin
Use in Specific Populations
Pregnancy
Teratogenic Effects
- When administered intramuscularly as the sodium salt at a dose of 1 gm to pregnant women, fosfomycin crosses the placental barrier. Fosfomycin crosses the placental barrier of rats; it does not produce teratogenic effects in pregnant rats at dosages as high as 1000 mg/kg/day (approximately 9 and 1.4 times the human dose based on body weight and mg/m2, respectively). When administered to pregnant female rabbits at dosages as high as 1000 mg/kg/day (approximately 9 and 2.7 times the human dose based on body weight and mg/m2, respectively), fetotoxicities were observed. However, these toxicities were seen at maternally toxic doses and were considered to be due to the sensitivity of the rabbit to changes in the intestinal microflora resulting from the antibiotic administration. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fosfomycin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fosfomycin during labor and delivery.
Nursing Mothers
- It is not known whether fosfomycin tromethamine is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Fosfomycin, a decision should be made whether to discontinue nursing or to not administer the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in children age 12 years and under have not been established in adequate and well-controlled studies.
Geriatic Use
- Clinical studies of Fosfomycin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Fosfomycin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fosfomycin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Fosfomycin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Fosfomycin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fosfomycin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fosfomycin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Fosfomycin in the drug label
IV Compatibility
There is limited information regarding IV Compatibility of Fosfomycin in the drug label.
Overdosage
- In acute toxicology studies, oral administration of high doses of Fosfomycin up to 5 gm/kg were well-tolerated in mice and rats, produced transient and minor incidences of watery stools in rabbits, and produced diarrhea with anorexia in dogs occurring 2-3 days after single dose administration. These doses represent 50-125 times the human therapeutic dose.
- The following events have been observed in patients who have taken Fosfomycin in overdose: vestibular loss, impaired hearing, metallic taste, and general decline in taste perception. In the event of overdosage, treatment should be symptomatic and supportive.
Pharmacology

Mechanism of Action
- Fosfomycin (the active component of fosfomycin tromethamine) hasin vitroactivity against a broad range of gram-positive and gram-negative aerobic microorganisms which are associated with uncomplicated urinary tract infections. Fosfomycin is bactericidal in urine at therapeutic doses. The bactericidal action of fosfomycin is due to its inactivation of the enzyme enolpyruvyl transferase, thereby irreversibly blocking the condensation of uridine diphosphate-N-acetylglucosamine with p-enolpyruvate, one of the first steps in bacterial cell wall synthesis. It also reduces adherence of bacteria to uroepithelial cells.
- There is generally no cross-resistance between fosfomycin and other classes of antibacterial agents such as beta-lactams and aminoglycosides.
- Fosfomycin has been shown to be active against most strains of the following microorganisms, both in vitroand in clinical infections
Structure
- Fosfomycin (fosfomycin tromethamine) sachet contains fosfomycin tromethamine, a synthetic, broad spectrum, bactericidal antibiotic for oral administration. It is available as a single-dose sachet which contains white granules consisting of 5.631 grams of fosfomycin tromethamine (equivalent to 3 grams of fosfomycin), and the following inactive ingredients: mandarin flavor, orange flavor, saccharin, and sucrose. The contents of the sachet must be dissolved in water. Fosfomycin tromethamine, a phosphonic acid derivative, is available as (1R,2S)-(1,2-epoxypropyl)phosphonic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1). It is a white granular compound with a molecular weight of 259.2. Its empirical formula is C3H7O4P.C4H11NO3, and its chemical structure is as follows:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Fosfomycin in the drug label.
Pharmacokinetics
Absorption
- Fosfomycin tromethamine is rapidly absorbed following oral administration and converted to the free acid, fosfomycin. Absolute oral bioavailability under fasting conditions is 37%. After a single 3-gm dose of Fosfomycin, the mean (± 1 SD) maximum serum concentration (Cmax) achieved was 26.1 (± 9.1) μg/mL within 2 hours. The oral bioavailability of fosfomycin is reduced to 30% under fed conditions. Following a single 3-gm oral dose of Fosfomycin with a high-fat meal, the mean Cmaxachieved was 17.6 (± 4.4) μg/mL within 4 hours.
- Cimetidine does not affect the pharmacokinetics of fosfomycin when coadministered with Fosfomycin. Metoclopramide lowers the serum concentrations and urinary excretion of fosfomycin when coadministered with Fosfomycin. (SeePRECAUTIONS, DRUG INTERACTIONS)
Distribution
- The mean apparent steady-state volume of distribution (Vss) is 136.1 (±44.1) L following oral administration of Fosfomycin. Fosfomycin is not bound to plasma proteins.
- Fosfomycin is distributed to the kidneys, bladder wall, prostate, and seminal vesicles. Following a 50 mg/Kg dose of fosfomycin to patients undergoing urological surgery for bladder carcinoma, the mean concentration of fosfomycin in the bladder, taken at a distance from the neoplastic site, was 18.0 μg per gram of tissue at 3 hours after dosing. Fosfomycin has been shown to cross the placental barrier in animals and man.
Excretion
- Fosfomycin is excreted unchanged in both urine and feces. Following oral administration of Fosfomycin, the mean total body clearance (CLTB) and mean renal clearance (CLR) of fosfomycin were 16.9 (± 3.5) L/hr and 6.3 (± 1.7) L/hr, respectively. Approximately 38% of a 3-gm dose of Fosfomycin is recovered from urine, and 18% is recovered from feces. Following intravenous administration, the mean CLTBand mean CLRof fosfomycin were 6.1 (± 1.0) L/hr and 5.5 (±1.2) L/hr, respectively.
- A mean urine fosfomycin concentration of 706 (± 466) μg/mL was attained within 2-4 hours after a single oral 3-gm dose of Fosfomycin under fasting conditions. The mean urinary concentration of fosfomycin was 10 μg/mL in samples collected 72-84 hours following a single oral dose of Fosfomycin.
- Following a 3-gm dose of Fosfomycin administered with a high fat meal, a mean urine fosfomycin concentration of 537 (± 252) μg/mL was attained within 6-8 hours. Although the rate of urinary excretion of fosfomycin was reduced under fed conditions, the cumulative amount of fosfomycin excreted in the urine was the same, 1118 (± 201) mg (fed) vs. 1140 mg (± 238) (fasting). Further, urinary concentrations equal to or greaterthan 100 μg/mL were maintained for the same duration, 26 hours, indicating that Fosfomycin can be taken without regard to food.
- Following oral administration of Fosfomycin, the mean half-life for elimination (t1/2) is 5.7 (± 2.8) hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long term carcinogenicity studies in rodents have not been conducted because Fosfomycin is intended for single dose treatment in humans. Fosfomycin was not mutagenic or genotoxic in thein vitroAmes' bacterial reversion test, in cultured human lymphocytes, in Chinese hamster V79 cells, and thein vivomouse micronucleus assay. Fosfomycin did not affect fertility or reproductive performance in male and female rats.
Clinical Studies
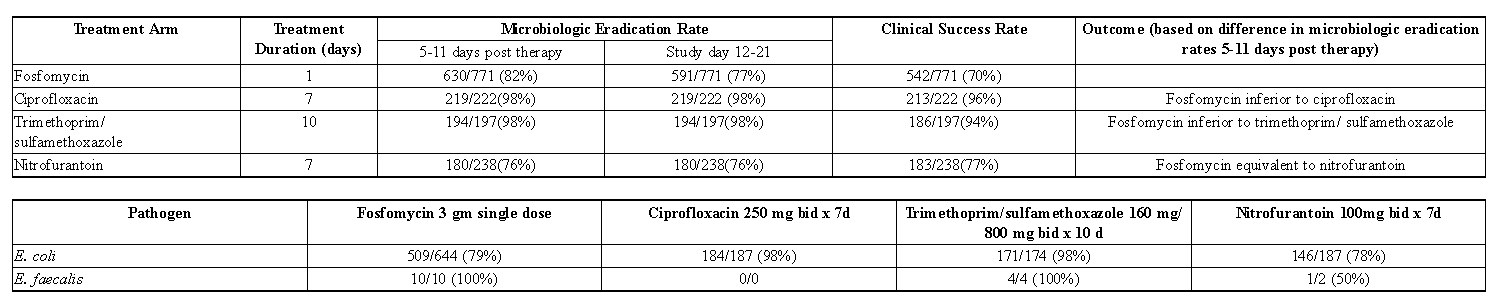
- In controlled, double-blind studies of acute cystitis performed in the United States, a single-dose of Fosfomycin was compared to three other oral antibiotics (See table below). The study population consisted of patients with symptoms and signs of acute cystitis of less than 4 days duration, no manifestations of upper tract infection (e.g., flank pain, chills, fever), no history of recurrent urinary tract infections (20% of patients in the clinical studies had a prior episode of acute cystitis within the preceding year), no known structural abnormalities, no clinical or laboratory evidence of hepatic dysfunction, and no known or suspected CNS disorders, such as epilepsy, or other factors which would predispose to seizures. In these studies, the following clinical success (resolution of symptoms) and microbiologic eradication rates were obtained.
How Supplied
- Fosfomycin is available as a single-dose sachet containing the equivalent of 3 grams of fosfomycin.
- NDC # 0456-4300-08
Storage
- Store at 25 C (77 F); excursions permitted to 15-30 C (59-86 F).
Images
Drug Images
{{#ask: Page Name::Fosfomycin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fosfomycin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- That Fosfomycin can be taken with or without food.
- That their symptoms should improve in two to three days after taking Fosfomycin; if not improved, the patient should contact her health care provider.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
- Alcohol-Fosfomycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Fosfomycin®
Look-Alike Drug Names
There is limited information regarding Fosfomycin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Fosfomycin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Fosfomycin |Label Name=Fosfomycin label01.png
}}
{{#subobject:
|Label Page=Fosfomycin |Label Name=Fosfomycin label02.png
}}