Foscarnet description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
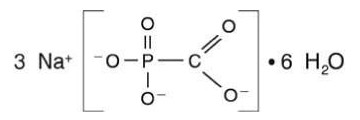
FOSCAVIR is the brand name for foscarnet sodium. The chemical name of foscarnet sodium is phosphonoformic acid, trisodium salt. Foscarnet sodium is a white, crystalline powder containing 6 equivalents of water of hydration with an empirical formula of Na3CO5P•6 H20 and a molecular weight of 300.1. The structural formula is:
 |
FOSCAVIR has the potential to chelate divalent metal ions, such as calcium and magnesium, to form stable coordination compounds. FOSCAVIR INJECTION is a sterile, isotonic aqueous solution for intravenous administration only. The solution is clear and colorless. Each milliliter of FOSCAVIR contains 24 mg of foscarnet sodium hexahydrate in Water for Injection, USP. Hydrochloric acid may have been added to adjust the pH of the solution to 7.4. FOSCAVIR INJECTION contains no preservatives.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020068s018lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.