Fluticasone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fluticasone is a corticosteroid that is FDA approved for the treatment of asthma, management of the nasal symptoms of seasonal and perennial allergic rhinitis and nonallergic rhinitis, inflammatory and pruritic manifestations of atopic dermatitis. Common adverse reactions include candidiasis of mouth and esophagus, nausea and vomiting, osteoporosis, headache, cough, epistaxis, pharyngitis, sinusitis, throat irritation, upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
The use of fluticasone is contraindicated in the following conditions:
- Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
- Hypersensitivity to any of the ingredients in the formulation.
Warnings
Aerosol
Local Effects
In clinical studies, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in patients treated with fluticasone. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral antifungal) therapy while treatment with fluticasone continues, but at times therapy with fluticasone may need to be interrupted. Patients should rinse the mouth after inhalation of fluticasone.
Acute Asthma Episodes
Fluticasone is not to be regarded as a bronchodilator and is not indicated for rapid relief of bronchospasm. Patients should be instructed to contact their physicians immediately when episodes of asthma that are not responsive to bronchodilators occur during the course of treatment with fluticasone. During such episodes, patients may require therapy with oral corticosteroids.
Immunosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered. Because of the potential for worsening infections, inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Transferring Patients From Systemic Corticosteroid Therapy
Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function. Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to fluticasone. In a clinical trial of 168 patients, prednisone reduction was successfully accomplished by reducing the daily prednisone dose on a weekly basis following initiation of treatment with fluticasone. Successive reduction of prednisone dose was allowed only when lung function, symptoms, and as-needed short-acting beta-agonist use were better than or comparable to that seen before initiation of prednisone dose reduction. Lung function (forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although inhaled corticosteroids may provide control of asthma symptoms during these episodes, in recommended doses they supply less than normal physiological amounts of glucocorticoid (cortisol) systemically and do NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Transfer of patients from systemic corticosteroid therapy to fluticasone may unmask conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions). Some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function).
Hypercorticism and Adrenal Suppression
Fluticasone propionate will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since fluticasone propionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of fluticasone in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing fluticasone.
Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with fluticasone should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response. It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients, particularly when fluticasone is administered at higher than recommended doses over prolonged periods of time. If such effects occur, the dosage of fluticasone should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids and for management of asthma.
Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and bronchospasm, may occur after administration of fluticasone.
Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long-term outcomes is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids), should be monitored and treated with established standards of care.
Effect on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving fluticasone routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms.
Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients following the long-term administration of inhaled corticosteroids, including fluticasone propionate. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Paradoxical Bronchospasm
As with other inhaled medications, bronchospasm may occur with an immediate increase in wheezing after dosing. If bronchospasm occurs following dosing with fluticasone, it should be treated immediately with a fast-acting inhaled bronchodilator. Treatment with fluticasone should be discontinued immediately and alternative therapy instituted.
Drug Interactions With Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone is not recommended because increased systemic corticosteroid adverse effects may occur.
Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other inhaled corticosteroids in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
Spray
- The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of withdrawal, e.g., joint and/or muscular pain, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
- The concomitant use of intranasal corticosteroids with other inhaled corticosteroids could increase the risk of signs or symptoms of hypercorticism and/or suppression of the HPA axis.
- A drug interaction study in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations.
- Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
- Avoid spraying in eyes.
Precautions
Spray
- Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients.
- Rarely, immediate hypersensitivity reactions or contact dermatitis may occur after the administration of fluticasone nasal spray. Rare instances of wheezing, nasal septum perforation, cataracts, glaucoma, and increased intraocular pressure have been reported following the intranasal application of corticosteroids, including fluticasone propionate.
- Use of excessive doses of corticosteroids may lead to signs or symptoms of hypercorticism and/or suppression of HPA function.
- Although systemic effects have been minimal with recommended doses of fluticasone nasal spray, potential risk increases with larger doses. Therefore, larger than recommended doses of fluticasone nasal spray should be avoided.
- When used at higher than recommended doses or in rare individuals at recommended doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of fluticasone nasal spray should be discontinued slowly consistent with accepted procedures for discontinuing oral corticosteroid therapy.
- In clinical studies with fluticasone propionate administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has occurred only rarely. When such an infection develops, it may require treatment with appropriate local therapy and discontinuation of treatment with fluticasone nasal spray. Patients using fluticasone nasal spray over several months or longer should be examined periodically for evidence of Candida infection or other signs of adverse effects on the nasal mucosa.
- Intranasal corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infections of the respiratory tract; untreated local or systemic fungal or bacterial infections; systemic viral or parasitic infections; or ocular herpes simplex.
- Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
Lotion/Cream
- Fluticasone contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Fluticasone should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
- Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal from treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
- Patients applying a potent topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. This may be done by using cosyntropin (ACTH1·24) stimulation testing.
- Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with fluticasone for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out. In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed.
- If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
- Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios.
- The following local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, hypertrichosis, and miliaria.
- Fluticasone, 0.05% may cause local cutaneous adverse reactions.
- If irritation develops, fluticasone should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
- If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of fluticasone should be discontinued until the infection has been adequately controlled.
- Fluticasone should not be used in the presence of preexisting skin atrophy and should not be used where infection is present at the treatment site. Fluticasone should not be used in the treatment of rosacea and perioral dermatitis.
- Patients that apply fluticasone to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.).
Adverse Reactions
Clinical Trials Experience
Aerosol
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The incidence of common adverse reactions in Table 2 is based upon 2 placebo-controlled US clinical trials in which 812 adult and adolescent patients (457 females and 355 males) previously treated with as-needed bronchodilators and/or inhaled corticosteroids were treated twice daily for up to 12 weeks with 2 inhalations of fluticasone 44 mcg Inhalation Aerosol,fluticasone 110 mcg Inhalation Aerosol,fluticasone 220 mcg Inhalation Aerosol (dosages of 88, 220, or 440 mcg twice daily), or placebo.
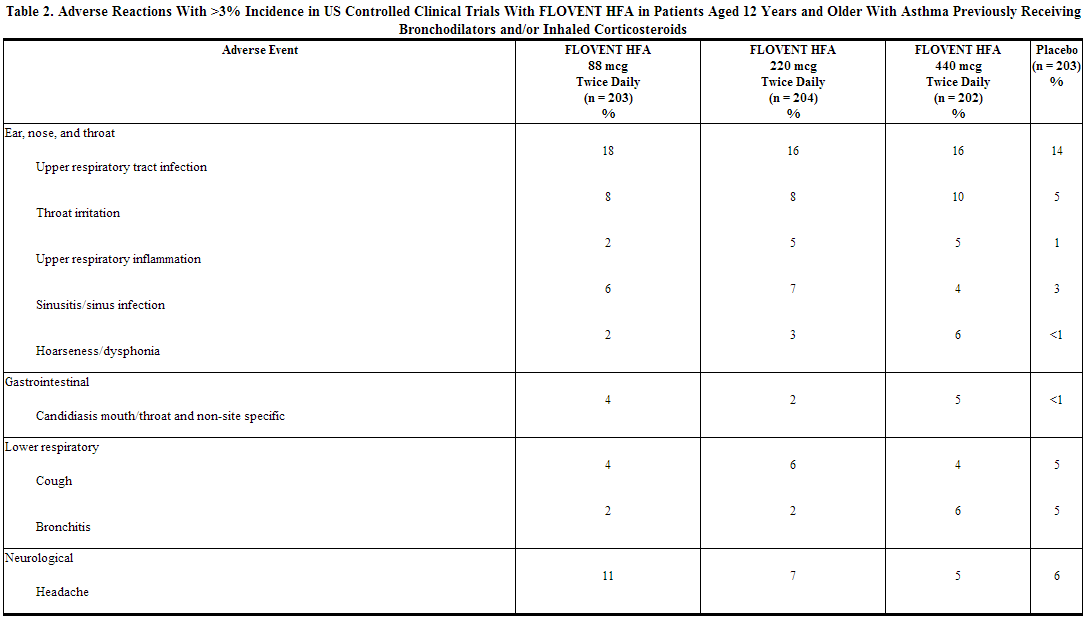
The table above includes all events (whether considered drug-related or nondrug-related by the investigator) that occurred at a rate of over 3% in any of the groups treated with fluticasone and were more common than in the placebo group. Less than 2% of patients discontinued from the studies because of adverse reactions. The average duration of exposure was 73 to 76 days in the active treatment groups compared with 60 days in the placebo group.
Additional Adverse Reactions: Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that were reported more frequently by patients with asthma treated with fluticasone compared with patients treated with placebo include the following: rhinitis, rhinorrhea/post-nasal drip, nasal sinus disorders, laryngitis, diarrhea, viral gastrointestinal infections, dyspeptic symptoms, gastrointestinal discomfort and pain, hyposalivation, musculoskeletal pain, muscle pain, muscle stiffness/tightness/rigidity, dizziness, migraines, fever, viral infections, pain, chest symptoms, viral skin infections, muscle injuries, soft tissue injuries, urinary infections.
Fluticasone propionate inhalation aerosol (440 or 880 mcg twice daily) was administered for 16 weeks to 168 patients with asthma requiring oral corticosteroids (Study 3). Adverse reactions not included above, but reported by more than 3 patients in either group treated with fluticasone and more commonly than in the placebo group included nausea and vomiting, arthralgia and articular rheumatism, and malaise and fatigue. In 2 long-term studies (26 and 52 weeks), the pattern of adverse reactions in patients treated with fluticasone at dosages up to 440 mcg twice daily was similar to that observed in the 12-week studies. There were no new and/or unexpected adverse reactions with long-term treatment. Pediatric Patients Aged 4 to 11 Years: fluticasone has been evaluated for safety in 56 pediatric patients who received 88 mcg twice daily for 4 weeks. Types of adverse reactions in these pediatric patients were generally similar to those observed in adults and adolescents.
Spray
In controlled US studies, more than 3,300 patients with seasonal allergic, perennial allergic, or perennial nonallergic rhinitis received treatment with intranasal fluticasone propionate. In general, adverse reactions in clinical studies have been primarily associated with irritation of the nasal mucous membranes, and the adverse reactions were reported with approximately the same frequency by patients treated with the vehicle itself. The complaints did not usually interfere with treatment. Less than 2% of patients in clinical trials discontinued because of adverse events; this rate was similar for vehicle placebo and active comparators.
Systemic corticosteroid side effects were not reported during controlled clinical studies up to 6 months’ duration with fluticasone nasal spray. If recommended doses are exceeded, however, or if individuals are particularly sensitive or taking fluticasone nasal spray in conjunction with administration of other corticosteroids, symptoms of hypercorticism, e.g., Cushing syndrome, could occur.
The following incidence of common adverse reactions (>3%, where incidence in fluticasone propionate-treated subjects exceeded placebo) is based upon 7 controlled clinical trials in which 536 patients (57 girls and 108 boys aged 4 to 11 years, 137 female and 234 male adolescents and adults) were treated with fluticasone nasal spray 200 mcg once daily over 2 to 4 weeks and 2 controlled clinical trials in which 246 patients (119 female and 127 male adolescents and adults) were treated with fluticasone nasal spray 200 mcg once daily over 6 months. Also included in the table are adverse events from 2 studies in which 167 children (45 girls and 122 boys aged 4 to 11 years) were treated with fluticasone nasal spray 100 mcg once daily for 2 to 4 weeks.
Overall Adverse Experiences With >3% Incidence on Fluticasone Propionate in Controlled Clinical Trials With fluticasone nasal spray in Patients ≥4 Years With Seasonal or Perennial Allergic Rhinitis.
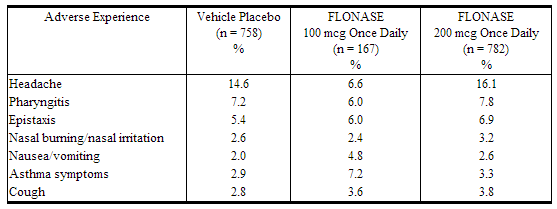
Other adverse events that occurred in ≤3% but ≥1% of patients and that were more common with fluticasone propionate (with uncertain relationship to treatment) included: blood in nasal mucus, runny nose, abdominal pain, diarrhea, fever, flu-like symptoms, aches and pains, dizziness, bronchitis.
Observed During Clinical Practice
In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of intranasal fluticasone propionate in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
- General: Hypersensitivity reactions, including angioedema, skin rash, edema of the face and tongue, pruritus, urticaria, bronchospasm, wheezing, dyspnea, and anaphylaxis/anaphylactoid reactions, which in rare instances were severe.
- Ear, Nose, and Throat: Alteration or loss of sense of taste and/or smell and, rarely, nasal septal perforation, nasal ulcer, sore throat, throat irritation and dryness, cough, hoarseness, and voice changes.
- Eye: Dryness and irritation, conjunctivitis, blurred vision, glaucoma, increased intraocular pressure, and cataracts.
- Cases of growth suppression have been reported for intranasal corticosteroids, including fluticasone.
Cream/Lotion
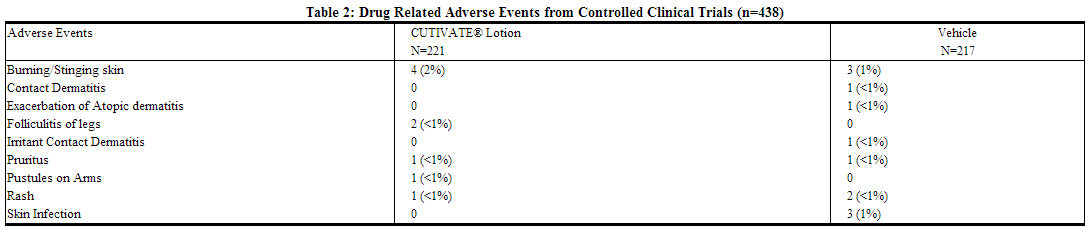
In 2 multicenter vehicle-controlled clinical trials of once-daily application of fluticasone by 196 adult and 242 pediatric patients, the total incidence of adverse reactions considered drug related by investigators was approximately 4%. Events were local cutaneous events, usually mild and self-limiting, and consisted primarily of burning/stinging (2%). All other drug-related events occurred with an incidence of less than 1%, and inclusively were contact dermatitis, exacerbation of atopic dermatitis, folliculitis of legs, pruritus, pustules on arm, rash, and skin infection. The incidence of drug-related events on drug compared to vehicle (4% and 5%, respectively) was similar. The incidence of drug-related events between study populations of 242 pediatric patients (age 3 months to < 17 years) and 196 adult patients (17 years or older) (4% and 5%, respectively) was also similar.
In an open-label study of 44 pediatric patients applying fluticasone to at least 35% of body surface area twice daily for 3 or 4 weeks, the overall incidence of drug-related adverse events was 14%. Events were local, cutaneous, and inclusively were dry skin (7%), stinging at application site (5%), and excoriation (2%).
The table below summarizes all adverse events by body system that occurred in at least 1% of patients in either the drug or vehicle group in controlled clinical trials.

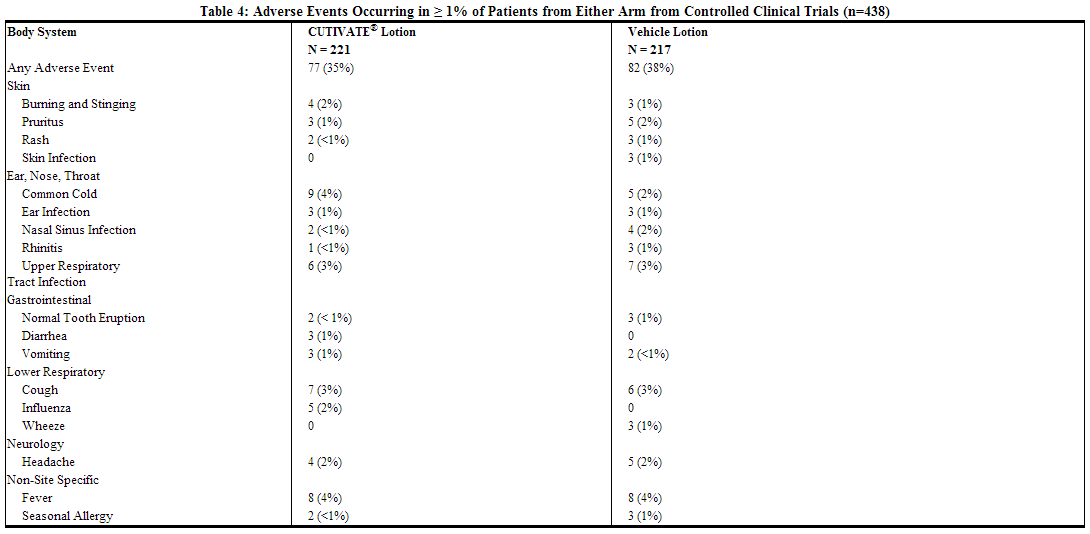
During the clinical trials, eczema herpeticum occurred in a 33-year-old male patient treated with fluticasone. Additionally, a 4-month-old patient treated with fluticasone in the open-label trial had marked elevations of the hepatic enzymes AST and ALT.
Postmarketing Experience
Aerosol
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postmarketing use of fluticasone propionate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
- Ear, Nose, and Throat: Aphonia, facial and oropharyngeal edema, and throat soreness and irritation.
- Endocrine and Metabolic: Cushingoid features, growth velocity reduction in children/adolescents, hyperglycemia, osteoporosis, and weight gain.
- Eye: Cataracts.
- Gastrointestinal Disorders: Dental caries and tooth discoloration.
- Immune System Disorders: Immediate and delayed hypersensitivity reactions, including urticaria, anaphylaxis, rash, and angioedema and bronchospasm, have been reported.
- Infections and Infestations: Esophageal candidiasis.
- Psychiatry: Agitation, aggression, anxiety, depression, and restlessness. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
- Respiratory: Asthma exacerbation, chest tightness, cough, dyspnea, immediate and delayed bronchospasm, paradoxical bronchospasm, pneumonia, and wheeze.
- Skin: Contusions, cutaneous hypersensitivity reactions, ecchymoses, and pruritus.
- Eosinophilic Conditions: In rare cases, patients on inhaled fluticasone propionate may present with systemic eosinophilic conditions, with some patients presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate
Spray
During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
Lotion/Cream
Systemic adverse events with fluticasone cream and fluticasone ointment have included:
- Immunosuppression/Pneumocystis carinii pneumonia/leukopenia/thrombocytopenia
- Hyperglycemia/ glycosuria
- Cushing syndrome
- Generalized body edema/blurred vision
- Acute urticarial reaction (edema, urticaria, pruritus, and throat swelling)
The following localized adverse reactions have been reported during post approval use of CUTIVATE® Lotion:
- Erythema
- Edema/swelling
- Bleeding
- Lack of efficacy
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
Aerosol
Strong Cytochrome P450 3A4 Inhibitors
Fluticasone propionate is a substrate of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone is not recommended because increased systemic corticosteroid adverse effects may occur.
A drug interaction study with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate concentration, resulting in significantly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9-fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol. Coadministration of fluticasone propionate and ketoconazole is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
Spray
Fluticasone propionate is a substrate of cytochrome P450 3A4. A drug interaction study with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.
In a placebo-controlled crossover study in 8 healthy volunteers, coadministration of a single dose of orally inhaled fluticasone propionate (1,000 mcg; 5 times the maximum daily intranasal dose) with multiple doses of ketoconazole (200 mg) to steady state resulted in increased plasma fluticasone propionate exposure, a reduction in plasma cortisol AUC, and no effect on urinary excretion of cortisol. Caution should be exercised when fluticasone nasal spray is coadministered with ketoconazole and other known potent cytochrome P450 3A4 inhibitors.
Use in Specific Populations
Pregnancy
Aerosol
There are no adequate and well-controlled studies with fluticasone in pregnant women. Fluticasone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects
Subcutaneous studies in mice at a dose approximately 0.1 times the maximum recommended human daily inhalation dose (MRHD) for adults on a mg/m2 basis and in the rat at a dose approximately 0.5 times the MRHD in adults on a mg/m2 basis revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification.
In rabbits, fetal weight reduction and cleft palate were observed at a subcutaneous dose approximately 0.04 times the MRHD for adults on a mg/m2 basis. However, no teratogenic effects were reported at oral doses up to approximately 3 times the MRHD for adults on a mg/m2 basis. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Spray
Subcutaneous studies in the mouse and rat at 45 and 100 mcg/kg, respectively (approximately equivalent to and 4 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m2 basis), revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification.
In the rabbit, fetal weight reduction and cleft palate were observed at a subcutaneous dose of 4 mcg/kg (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis). However, no teratogenic effects were reported at oral doses up to 300 mcg/kg (approximately 25 times the maximum recommended daily intranasal dose in adults on a mcg/m2 basis) of fluticasone propionate to the rabbit. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following oral administration of 100 mcg/kg to rats and 300 mcg/kg to rabbits (approximately 4 and 25 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m2 basis).
There are no adequate and well-controlled studies in pregnant women. Fluticasone propionate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Lotion/Cream
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Systemic embryofetal development studies were conducted in mice, rats and rabbits. Subcutaneous doses of 15, 45 and 150 μg/kg/day of fluticasone propionate were administered to pregnant female mice from gestation days 6 – 15. A teratogenic effect characteristic of corticosteroids (cleft palate) was noted after administration of 45 and 150 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 15 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 10, 30 and 100 μg/kg/day of fluticasone propionate were administered to pregnant female rats in two embryofetal development studies (one study administered fluticasone propionate from gestation days 6 – 15 and the other study from gestation days 7 – 17). In the presence of maternal toxicity, fetal effects noted at 100 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, omphalocele, cleft palate, and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 10 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 0.08, 0.57 and 4 μg/kg/day of fluticasone propionate were administered to pregnant female rabbits from gestation days 6 – 18. Fetal effects noted at 4 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, cleft palate and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.57 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Oral doses of 3, 30 and 300 μg/kg/day fluticasone propionate were administered to pregnant female rabbits from gestation days 8 – 20. No fetal or teratogenic effects were noted at oral doses up to 300 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. However, no fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
There are no adequate and well-controlled studies in pregnant women. During clinical trials of fluticasone, women of childbearing potential were required to use contraception to avoid pregnancy. Therefore, fluticasone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fluticasone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fluticasone during labor and delivery.
Nursing Mothers
Aerosol
It is not known whether fluticasone propionate is excreted in human breast milk. However, other corticosteroids have been detected in human milk. Subcutaneous administration to lactating rats of tritiated fluticasone propionate (approximately 0.05 times the MRHD in adults on a mg/m2 basis) resulted in measurable radioactivity in milk. Since there are no data from controlled trials on the use of FLOVENT HFA by nursing mothers, caution should be exercised when FLOVENT HFA is administered to a nursing woman.
Spray
Subcutaneous administration to lactating rats of 10 mcg/kg of tritiated fluticasone propionate (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis) resulted in measurable radioactivity in the milk. Since there are no data from controlled trials on the use of intranasal fluticasone propionate by nursing mothers, caution should be exercised when FLONASE Nasal Spray is administered to a nursing woman.
Lotion/Cream
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when CUTIVATE® Lotion is administered to a nursing woman.
Pediatric Use
Aerosol
The safety and effectiveness of FLOVENT HFA in children aged 4 years and older have been established [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.2)]. The safety and effectiveness of FLOVENT HFA in children younger than 4 years have not been established. Use of FLOVENT HFA in patients aged 4 to 11 years is supported by evidence from adequate and well-controlled studies in adults and adolescents aged 12 years and older, pharmacokinetic studies in patients aged 4 to 11 years, established efficacy of fluticasone propionate formulated as FLOVENT® DISKUS® (fluticasone propionate inhalation powder) and FLOVENT® ROTADISK® (fluticasone propionate inhalation powder) in patients aged 4 to 11 years, and supportive findings with FLOVENT HFA in a study conducted in patients aged 4 to 11 years.
Effects on Growth: Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. A reduction of growth velocity in children or teenagers may occur as a result of poorly controlled asthma or from use of corticosteroids including inhaled corticosteroids. The effects of long-term treatment of children and adolescents with inhaled corticosteroids, including fluticasone propionate, on final adult height are not known. Controlled clinical studies have shown that inhaled corticosteroids may cause a reduction in growth in pediatric patients. In these studies, the mean reduction in growth velocity was approximately 1 cm/year (range: 0.3 to 1.8 cm/year) and appeared to depend upon dose and duration of exposure. This effect was observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The effects on growth velocity of treatment with orally inhaled corticosteroids for over 1 year, including the impact on final adult height, are unknown. The growth of children and adolescents receiving orally inhaled corticosteroids, including FLOVENT HFA, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including FLOVENT HFA, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
Since a cross study comparison in adult and adolescent patients (aged 12 years and older) indicated that systemic exposure of inhaled fluticasone propionate from FLOVENT HFA would be higher than exposure from FLOVENT ROTADISK, results from a study to assess the potential growth effects of FLOVENT ROTADISK in pediatric patients (aged 4 to 11 years) are provided.
A 52-week placebo-controlled study to assess the potential growth effects of fluticasone propionate inhalation powder (FLOVENT ROTADISK) at 50 and 100 mcg twice daily was conducted in the US in 325 prepubescent children (244 males and 81 females) aged 4 to 11 years. The mean growth velocities at 52 weeks observed in the intent-to-treat population were 6.32 cm/year in the placebo group (n = 76), 6.07 cm/year in the 50-mcg group (n = 98), and 5.66 cm/year in the 100-mcg group (n = 89). An imbalance in the proportion of children entering puberty between groups and a higher dropout rate in the placebo group due to poorly controlled asthma may be confounding factors in interpreting these data. A separate subset analysis of children who remained prepubertal during the study revealed growth rates at 52 weeks of 6.10 cm/year in the placebo group (n = 57), 5.91 cm/year in the 50-mcg group (n = 74), and 5.67 cm/year in the 100-mcg group (n = 79). In children aged 8.5 years, the mean age of children in this study, the range for expected growth velocity is: boys – 3rd percentile = 3.8 cm/year, 50th percentile = 5.4 cm/year, and 97th percentile = 7.0 cm/year; girls – 3rd percentile = 4.2 cm/year, 50th percentile = 5.7 cm/year, and 97th percentile = 7.3 cm/year. The clinical significance of these growth data is not certain. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route, and weigh the benefits of corticosteroid therapy against the possibility of growth suppression if growth appears slowed. Patients should be maintained on the lowest dose of inhaled corticosteroid that effectively controls their asthma.
Children Younger Than 4 Years:Pharmacokinetics: Pharmacodynamics: A 12-week, double-blind, placebo-controlled, parallel-group study was conducted in children with asthma aged 1 to younger than 4 years. Twelve-hour overnight urinary cortisol excretion after a 12-week treatment period with 88 mcg of FLOVENT HFA twice daily (n = 73) and with placebo (n = 42) were calculated. The mean and median change from baseline in urine cortisol over 12 hours were -0.7 and 0.0 mcg for FLOVENT HFA and 0.3 and -0.2 mcg for placebo, respectively.
In a 1-way crossover study in children aged 6 to younger than 12 months with reactive airways disease (N = 21), serum cortisol was measured over a 12-hour dosing period. Patients received placebo treatment for a 2-week period followed by a 4-week treatment period with 88 mcg of FLOVENT HFA twice daily with an AeroChamber Plus® Valved Holding Chamber (VHC) with mask. The geometric mean ratio of serum cortisol over 12 hours (AUC0-12 h) following FLOVENT HFA (n = 16) versus placebo (n = 18) was 0.95 (95% CI: 0.72, 1.27).
Safety: FLOVENT HFA administered as 88 mcg twice daily has been evaluated for safety in 239 pediatric patients aged 1 to younger than 4 years in a 12-week, double-blind, placebo-controlled study. Treatments were administered with an AeroChamber Plus VHC with mask. In pediatric patients aged 1 to younger than 4 years receiving FLOVENT HFA, the following events occurred with a frequency greater than 3% and more frequently than in pediatric patients who received placebo, regardless of causality assessment: pyrexia, nasopharyngitis, upper respiratory tract infection, vomiting, otitis media, diarrhea, bronchitis, pharyngitis, and viral infection.
FLOVENT HFA administered as 88 mcg twice daily has also been evaluated for safety in 23 pediatric patients aged 6 to 12 months in an open-label placebo-controlled study. Treatments were administered with an AeroChamber Plus VHC with mask for 2 weeks with placebo followed by 4 weeks with active drug. There was no discernable difference in the types of adverse events reported between patients receiving placebo compared to the active drug.
In Vitro Testing of Dose Delivery With Holding Chambers: In vitro dose characterization studies were performed to evaluate the delivery of FLOVENT HFA via holding chambers with attached masks. The studies were conducted with 2 different holding chambers (AeroChamber Plus VHC and AeroChamber Z-STAT Plus™ VHC) with masks (small and medium size) at inspiratory flow rates of 4.9, 8.0, and 12.0 L/min in combination with holding times of 0, 2, 5, and 10 seconds. The flow rates were selected to be representative of inspiratory flow rates of children aged 6 to 12 months, 2 to 5 years, and over 5 years, respectively. The mean delivered dose of fluticasone propionate through the holding chambers with masks was lower than the 44 mcg of fluticasone propionate delivered directly from the actuator mouthpiece. The results were similar through both holding chambers (see Table 3 for data for the AeroChamber Plus VHC). The fine particle fraction (approximately 1 to 5 μm) across the flow rates used in these studies was 70% to 84% of the delivered dose, consistent with the removal of the coarser fraction by the holding chamber. In contrast, the fine particle fraction for FLOVENT HFA delivered without a holding chamber typically represents 42% to 55% of the delivered dose measured at the standard flow rate of 28.3 L/min. These data suggest that, on a per kilogram basis, young children receive a comparable dose of fluticasone propionate when delivered via a holding chamber and mask as adults do without their use.
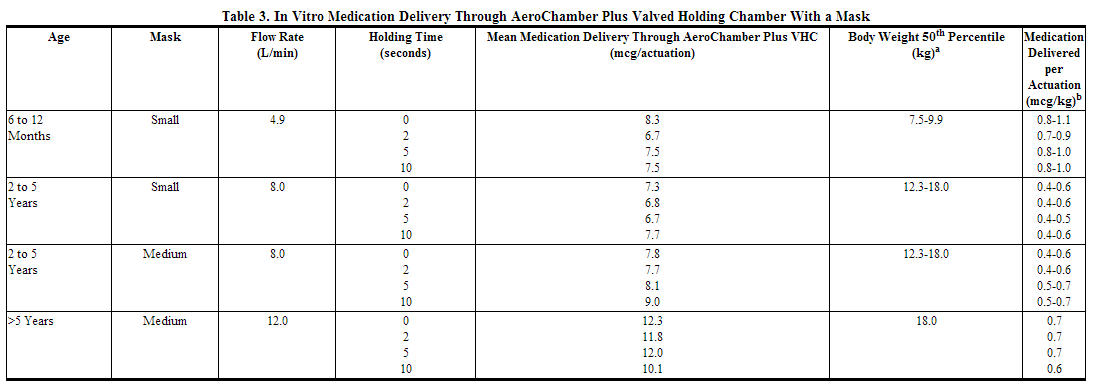
aCenters for Disease Control growth charts, developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000). Ranges correspond to the average of the 50th percentile weight for boys and girls at the ages indicated. bA single inhalation of FLOVENT HFA in a 70-kg adult without use of a valved holding chamber and mask delivers approximately 44 mcg, or 0.6 mcg/kg.
Spray
Six hundred fifty (650) patients aged 4 to 11 years and 440 patients aged 12 to 17 years were studied in US clinical trials with fluticasone propionate nasal spray. The safety and effectiveness of FLONASE Nasal Spray in children below 4 years of age have not been established.
Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including FLONASE Nasal Spray, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks/benefits of treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including FLONASE Nasal Spray, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A 1-year placebo-controlled clinical growth study was conducted in 150 pediatric patients (ages 3 to 9 years) to assess the effect of FLONASE Nasal Spray (single daily dose of 200 mcg, the maximum approved dose) on growth velocity. From the primary population of 56 patients receiving FLONASE Nasal Spray and 52 receiving placebo, the point estimate for growth velocity with FLONASE Nasal Spray was 0.14 cm/year lower than that noted with placebo (95% confidence interval ranging from 0.54 cm/year lower than placebo to 0.27 cm/year higher than placebo). Thus, no statistically significant effect on growth was noted compared to placebo. No evidence of clinically relevant changes in HPA axis function or bone mineral density was observed as assessed by 12-hour urinary cortisol excretion and dual-energy x-ray absorptiometry, respectively.
The potential for FLONASE Nasal Spray to cause growth suppression in susceptible patients or when given at higher doses cannot be ruled out.
Lotion/Cream
CUTIVATE® Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. CUTIVATE® Lotion should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis. CUTIVATE® Lotion should be discontinued if control is achieved before 4 weeks. If no improvement is seen within 2 weeks, contact a physician. The safety of the use of CUTIVATE® Lotion for longer than 4 weeks has not been established.
The safety and efficacy of CUTIVATE® Lotion in pediatric patients below 1 year of age have not been established. Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by the physician. CUTIVATE® Lotion should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with CUTIVATE® Lotion for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out.
In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed. CUTIVATE® (fluticasone propionate) Cream, 0.05% caused HPA axis suppression in 2 of 43 pediatric patients, ages 2 and 5 years old, who were treated for 4 weeks covering at least 35% of the body surface area. Follow-up testing 12 days after treatment discontinuation, available for 1 of the 2 patients, demonstrated a normally responsive HPA axis.
HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include low plasma cortisol levels to an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
In addition, local adverse events including cutaneous atrophy, striae, telangiectasia, and pigmentation change have been reported with topical use of corticosteroids in pediatric patients.
Geriatic Use
Aerosol
Of the total number of patients treated with FLOVENT HFA in US and non-US clinical trials, 173 were aged 65 years or older, 19 of which were 75 years or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Spray
A limited number of patients 65 years of age and older (n = 129) or 75 years of age and older (n = 11) have been treated with FLONASE Nasal Spray in US and non-US clinical trials. While the number of patients is too small to permit separate analysis of efficacy and safety, the adverse reactions reported in this population were similar to those reported by younger patients.
Lotion/Cream
A limited number of patients above 65 years of age have been treated with CUTIVATE® Lotion in US and non-US clinical trials. Specifically only 8 patients above 65 years of age were treated with CUTIVATE® Lotion in controlled clinical trials. The number of patients is too small to permit separate analyses of efficacy and safety.
Gender
There is no FDA guidance on the use of Fluticasone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fluticasone with respect to specific racial populations.
Renal Impairment
Formal pharmacokinetic studies using FLOVENT HFA have not been conducted in patients with renal impairment.
Hepatic Impairment
Formal pharmacokinetic studies using FLOVENT HFA have not been conducted in patients with hepatic impairment. Since fluticasone propionate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate in plasma. Therefore, patients with hepatic disease should be closely monitored.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fluticasone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fluticasone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhaled
- Spray
- Cutaneous application
Monitoring
Transferring Patients From Systemic Corticosteroid Therapy
Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress.
Aerosol
Lung function (forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids.
IV Compatibility
There is limited information regarding the compatibility of Fluticasone and IV administrations.
Overdosage
- Chronic overdosage may result in signs/symptoms of hypercorticism.
- Inhalation by healthy volunteers of a single dose of 1,760 or 3,520 mcg of fluticasone was well tolerated. Doses of 1,320 mcg administered to healthy human volunteers twice daily for 7 to 15 days were also well tolerated.
- Intranasal administration of 2 mg (10 times the recommended dose) of fluticasone propionate twice daily for 7 days to healthy human volunteers was well tolerated. Single oral doses up to 16 mg have been studied in human volunteers with no acute toxic effects reported.
- Repeat oral doses up to 80 mg daily for 10 days in healthy volunteers and repeat oral doses up to 20 mg daily for 42 days in patients were well tolerated. Adverse reactions were of mild or moderate severity, and incidences were similar in active and placebo treatment groups.
- No deaths were seen in mice given an oral dose of 1,000 mg/kg (approximately 2,300 and 11,000 times the MRHD for adults and children aged 4 to 11 years, respectively, on a mg/m2 basis). No deaths were seen in rats given an oral dose of 1,000 mg/kg (approximately 4,600 and 22,000 times the MRHD in adults and children aged 4 to 11 years, respectively, on a mg/m2 basis).
- The oral and subcutaneous median lethal doses in mice and rats were >1,000 mg/kg (>20,000 and >41,000 times, respectively, the maximum recommended daily intranasal dose in adults and >10,000 and >20,000 times, respectively, the maximum recommended daily intranasal dose in children on a mg/m2 basis).
- Topically applied fluticasone can be absorbed in sufficient amounts to produce systemic effects.
Pharmacology
Fluticasone
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Fluticasone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Fluticasone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fluticasone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Fluticasone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Fluticasone Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.