Fluorouracil (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Fluorouracil (injection) is an antineoplastic-agent that is FDA approved for the treatment of carcinoma of the colon, rectum, breast, stomach and pancreas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include stomatitis and esophagopharyngitis (which may lead to sloughing and ulceration), diarrhea, anorexia, Leukopenia, nausea and emesis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Carcinoma of the colon, rectum, breast, stomach and pancreas
- Fluorouracil Injection is effective in the palliative management of carcinoma of the colon, rectum, breast, stomach and pancreas.
Dosing Information
General Instructions
- Fluorouracil Injection should be administered only intravenously, using care to avoid extravasation. No dilution is required.
- All dosages are based on the patient's actual weight. However, the estimated lean body mass (dry weight) is used if the patient is obese or if there has been a spurious weight gain due to edema, ascites or other forms of abnormal fluid retention.
- It is recommended that prior to treatment each patient be carefully evaluated in order to estimate as accurately as possible the optimum initial dosage of Fluorouracil .
Dosage
- Twelve mg/kg are given intravenously once daily for four successive days. The daily dose should not exceed 800 mg. If no toxicity is observed, 6 mg/kg are given on the 6th, 8th, 10th and 12th days unless toxicity occurs. No therapy is given on the 5th, 7th, 9th or 11th days. Therapy is to be discontinued at the end of the 12th day, even if no toxicity has become apparent.
- Poor risk patients or those who are not in an adequate nutritional state should receive 6 mg/kg/day for three days. If no toxicity is observed, 3 mg/kg may be given on the 5th, 7 th and 9 th days unless toxicity occurs. No therapy is given on the 4 th, 6 th or 8 th days. The daily dose should not exceed 400 mg.
- A sequence of injections on either schedule constitutes a "course of therapy."
Maintenance Therapy
- In instances where toxicity has not been a problem, it is recommended that therapy be continued using either of the following schedules:
- Repeat dosage of first course every 30 days after the last day of the previous course of treatment.
- When toxic signs resulting from the initial course of therapy have subsided, administer a maintenance dosage of 10 to 15 mg/kg/week as a single dose. Do not exceed 1 g per week.
- The patient's reaction to the previous course of therapy should be taken into account in determining the amount of the drug to be used, and the dosage should be adjusted accordingly. Some patients have received from 9 to 45 courses of treatment during periods which ranged from 12 to 60 months.
Handling and Disposal
- Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1–7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
- Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If a precipitate occurs due to exposure to low temperatures, resolubilize by heating to 140°F and shaking vigorously; allow to cool to body temperature before using.
Directions for proper use of pharmacy bulk package
(Not for Direct Infusion)
- The 50 mL and 100 mL Pharmacy Bulk Packages are for use in a Pharmacy Admixture Service only. They should be inserted into a plastic hanging device and suspended as a unit in a laminar flow hood. Use only if clear and seal is intact and undamaged.
- Use of this product is restricted to a suitable work area, such as a laminar flow hood. Use only if clear and seal is intact and undamaged. Prior to entering the vial, remove the flip-off seal and cleanse the rubber closure with a suitable antiseptic agent. The container closure may be penetrated only one time, utilizing a suitable sterile transfer device or dispensing set which allows measured distribution of the contents. The date and time the vial was initially opened should be recorded in the space provided on the vial label. Transfer individual dose(s) to appropriate intravenous infusion solutions. Use of a syringe with a needle is not recommended. Multiple entries increase the potential of microbial and particulate contamination.
- The withdrawal of container contents should be accomplished without delay using aseptic technique.However, should this not be possible, a maximum time of 4 hours from initial closure entry is permitted to complete fluid transfer operations. It is recommended that the transferred fluids be used promptly.
Recommended Storage Conditions After Opening
- Keep under laminar flow hood at room temperature.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluorouracil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluorouracil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- Safety and effectiveness in children have not been established.
Non–Guideline-Supported Use
- Safety and effectiveness in children have not been established.
Contraindications
- Fluorouracil Injection therapy is contraindicated for patients in a poor nutritional state, those with depressed bone marrow function, those with potentially serious infections or those with a known hypersensitivity to Fluorouracil .
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- THE DAILY DOSE OF Fluorouracil INJECTION IS NOT TO EXCEED 800 MG. IT IS RECOMMENDED THAT PATIENTS BE HOSPITALIZED DURING THEIR FIRST COURSE OF TREATMENT.
- Fluorouracil should be used with extreme caution in poor risk patients with a history of high-dose pelvic irradiation or previous use of alkylating agents, those who have a widespread involvement of bone marrow by metastatic tumors or those with impaired hepatic or renal function.
- Rarely, unexpected, severe toxicity (e.g., stomatitis, diarrhea, neutropenia and neurotoxicity) associated with 5-fluorouracil has been attributed to deficiency of dipyrimidine dehydrogenase activity. A few patients have been rechallenged with 5-fluorouracil and despite 5-fluorouracil dose lowering, toxicity recurred and progressed with worse morbidity. Absence of this catabolic enzyme appears to result in prolonged clearance of 5-fluorouracil.
Combination Therapy
- Any form of therapy which adds to the stress of the patient, interferes with nutrition or depresses bone marrow function will increase the toxicity of fluorouracil.
Precautions
General
- Fluorouracil is a highly toxic drug with a narrow margin of safety. Therefore, patients should be carefully supervised, since therapeutic response is unlikely to occur without some evidence of toxicity. Severe hematological toxicity, gastrointestinal hemorrhage and even death may result from the use of fluorouracil despite meticulous selection of patients and careful adjustment of dosage. Although severe toxicity is more likely in poor risk patients, fatalities may be encountered occasionally even in patients in relatively good condition.
- Therapy is to be discontinued promptly whenever one of the following signs of toxicity appears:
- Stomatitis or esophagopharyngitis, at the first visible sign.
- Leukopenia (WBC under 3500) or a rapidly falling white blood count.
- Vomiting, intractable.
- Diarrhea, frequent bowel movements or watery stools.
- Gastrointestinal ulceration and bleeding.
- Thrombocytopenia (platelets under 100,000).
- Hemorrhage from any site.
- The administration of 5-fluorouracil has been associated with the occurrence of Palmar planter erythrodysesthesia syndrome, also known as hand-foot syndrome. This syndrome has been characterized as a tingling sensation of hands and feet which may progress over the next few days to pain when holding objects or walking. The palms and soles become symmetrically swollen and erythematous with tenderness of the distal phalanges, possibly accompanied by desquamation. Interruption of therapy is followed by gradual resolution over 5 to 7 days. Although pyroxine has been reported to ameliorate the Palmar planter erythrodysesthesia syndrome, its safety and effectiveness have not been established.
Adverse Reactions
Clinical Trials Experience
- Stomatitis and esophagopharyngitis (which may lead to sloughing and ulceration), diarrhea, anorexia, nausea and emesis are commonly seen during therapy.
- Leukopenia usually follows every course of adequate therapy with fluorouracil. The lowest white blood cell counts are commonly observed between the 9th and 14th days after the first course of treatment, although uncommonly the maximal depression may be delayed for as long as 20 days. By the 30th day the count has usually returned to the normal range.
- Alopecia and dermatitis may be seen in a substantial number of cases. The dermatitis most often seen is a pruritic maculopapular rash usually appearing on the extremities and less frequently on the trunk. It is generally reversible and usually responsive to symptomatic treatment.
- Other adverse reactions are:
- Hematologic: pancytopenia, thrombocytopenia, agranulocytosis, anemia.
- Cardiovascular: myocardial ischemia, angina.
- Gastrointestinal: gastrointestinal ulceration and bleeding.
- Allergic Reactions: anaphylaxis and generalized allergic reactions.
- Neurologic: acute cerebellar syndrome (which may persist following discontinuance of treatment), nystagmus, headache.
- Dermatologic: dry skin; fissuring; photosensitivity, as manifested by erythema or increased pigmentation of the skin; vein pigmentation;Palmar planter erythrodysesthesia syndrome, as manifested by tingling of the hands and feet followed by pain, erythema and swelling.
- Ophthalmic: lacrimal duct stenosis, visual changes, lacrimation, photophobia.
- Psychiatric: disorientation, confusion, euphoria.
- Miscellaneous: thrombophlebitis, epistaxis, nail changes (including loss of nails).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Fluorouracil in the drug label.
Drug Interactions
- Leucovorin calcium may enhance the toxicity of fluorouracil.
Use in Specific Populations
Pregnancy
- Fluorouracil may cause fetal harm when administered to a pregnant woman. Fluorouracil has been shown to be teratogenic in laboratory animals. Fluorouracil exhibited maximum teratogenicity when given to mice as single intraperitoneal injections of 10 to 40 mg/kg on day 10 or 12 of gestation. Similarly, intraperitoneal doses of 12 to 37 mg/kg given to rats between days 9 and 12 of gestation and intramuscular doses of 3 to 9 mg given to hamsters between days 8 and 11 of gestation were teratogenic. Malformations included cleft palates, skeletal defects and deformed appendages, paws and tails. The dosages which were teratogenic in animals are 1 to 3 times the maximum recommended human therapeutic dose. In monkeys, divided doses of 40 mg/kg given between days 20 and 24 of gestation were not teratogenic.
- There are no adequate and well-controlled studies with fluorouracil in pregnant women. While there is no evidence of teratogenicity in humans due to fluorouracil, it should be kept in mind that other drugs which inhibit DNA synthesis (e.g., methotrexate and aminopterin) have been reported to be teratogenic in humans. Women of childbearing potential should be advised to avoid becoming pregnant.
- If the drug is used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be told of the potential hazard to the fetus. Fluorouracil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
- Fluorouracil has not been studied in animals for its effects on peri- and postnatal development. However, fluorouracil has been shown to cross the placenta and enter into fetal circulation in the rat. Administration of fluorouracil has resulted in increased resorptions and embryolethality in rats. In monkeys, maternal doses higher than 40 mg/kg resulted in abortion of all embryos exposed to fluorouracil. Compounds which inhibit DNA, RNA and protein synthesis might be expected to have adverse effects on peri- and postnatal development.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fluorouracil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fluorouracil during labor and delivery.
Nursing Mothers
- It is not known whether fluorouracil is excreted in human milk. Because fluorouracil inhibits DNA, RNA and protein synthesis, mothers should not nurse while receiving this drug.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Fluorouracil with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Fluorouracil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fluorouracil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Fluorouracil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Fluorouracil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fluorouracil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fluorouracil in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- White blood counts with differential are recommended before each dose.
IV Compatibility
There is limited information regarding IV Compatibility of Fluorouracil in the drug label.
Overdosage
- The possibility of overdosage with fluorouracil is unlikely in view of the mode of administration. Nevertheless, the anticipated manifestations would be nausea, vomiting, diarrhea, gastrointestinal ulceration and bleeding, bone marrow depression (including thrombocytopenia, leukopenia and agranulocytosis). No specific antidotal therapy exists. Patients who have been exposed to an overdose of fluorouracil should be monitored hematologically for at least four weeks. Should abnormalities appear, appropriate therapy should be utilized.
- The acute intravenous toxicity of fluorouracil is as follows:
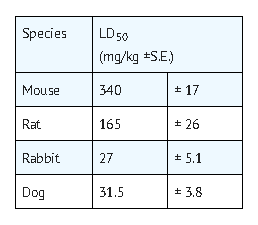
Pharmacology
Mechanism of Action
- There is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner, fluorouracil interferes with the synthesis of deoxyribonucleic acid (DNA) and to a lesser extent inhibits the formation of ribonucleic acid (RNA). Since DNA and RNA are essential for cell division and growth, the effect of fluorouracil may be to create a thymine deficiency which provokes unbalanced growth and death of the cell. The effects of DNA and RNA deprivation are most marked on those cells which grow more rapidly and which take up fluorouracil at a more rapid rate.
Structure
- Fluorouracil ® Injection (fluorouracil injection, USP) an antineoplastic antimetabolite, is a colorless to faint yellow aqueous, sterile, nonpyrogenic injectable solution available in a 50 mL and 100 mL Pharmacy Bulk Package for intravenous administration. Each mL contains 50 mg fluorouracil in water for injection, USP, pH is adjusted to 8.6 to 9.4 with sodium hydroxide.
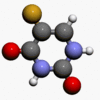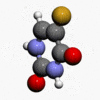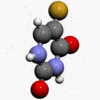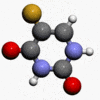
- Chemically, fluorouracil, a fluorinated pyrimidine, is 5-fluoro-2,4 (1H,3H)-pyrimidinedione. It is a white to practically white crystalline powder which is sparingly soluble in water. The structural formula is:
- Molecular formula: C4H3FN2O2
- Molecular weight 130.08
- A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Fluorouracil in the drug label.
Pharmacokinetics
- Following intravenous injection, fluorouracil distributes into tumors, intestinal mucosa, bone marrow, liver and other tissues throughout the body. In spite of its limited lipid solubility, fluorouracil diffuses readily across the blood-brain barrier and distributes into cerebrospinal fluid and brain tissue.
- Seven to twenty percent of the parent drug is excreted unchanged in the urine in six hours; of this over 90% is excreted in the first hour. The remaining percentage of the administered dose is metabolized, primarily in the liver. The catabolic metabolism of fluorouracil results in degradation products (e.g., CO2, urea and α-fluoro-ß-alanine) which are inactive. The inactive metabolites are excreted in the urine over the next 3 to 4 hours. When fluorouracil is labeled in the six carbon position, thus preventing the 14C metabolism to CO2, approximately 90% of the total radioactivity is excreted in the urine. When fluorouracil is labeled in the two carbon position approximately 90% of the total radioactivity is excreted in expired CO2. Ninety percent of the dose is accounted for during the first 24 hours following intravenous administration.
- Following intravenous administration of fluorouracil, the mean half-life of elimination from plasma is approximately 16 minutes, with a range of 8 to 20 minutes, and is dose dependent. No intact drug can be detected in the plasma 3 hours after an intravenous injection.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Long-term studies in animals to evaluate the carcinogenic potential of fluorouracil have not been conducted. However, there was no evidence of carcinogenicity in small groups of rats given fluorouracil orally at doses of 0.01, 0.3, 1 or 3 mg per rat 5 days per week for 52 weeks, followed by a six-month observation period. Also, in other studies, 33 mg/kg of fluorouracil was administered intravenously to male rats once a week for 52 weeks followed by observation for the remainder of their lifetimes with no evidence of carcinogenicity. Female mice were given 1 mg of fluorouracil intravenously once a week for 16 weeks with no effect on the incidence of lung adenomas. On the basis of the available data, no evaluation can be made of the carcinogenic risk of fluorouracil to humans.
Mutagenesis
- Oncogenic transformation of fibroblasts from mouse embryo has been induced in vitro by fluorouracil, but the relationship between oncogenicity and mutagenicity is not clear. Fluorouracil has been shown to be mutagenic to several strains of Salmonella typhimurium, including TA 1535, TA 1537 and TA 1538, and to Saccharomyces cerevisiae, although no evidence of mutagenicity was found with Salmonella typhimurium strains TA 92, TA 98 and TA 100. In addition, a positive effect was observed in the micronucleus test on bone marrow cells of the mouse, and fluorouracil at very high concentrations produced chromosomal breaks in hamster fibroblasts in vitro.
Impairment of Fertility
- Fluorouracil has not been adequately studied in animals to permit an evaluation of its effects on fertility and general reproductive performance. However, doses of 125 or 250 mg/kg, administered intraperitoneally, have been shown to induce chromosomal aberrations and changes in chromosomal organization of spermatogonia in rats. Spermatogonial differentiation was also inhibited by fluorouracil, resulting in transient infertility. However, in studies with a strain of mouse which is sensitive to the induction of sperm head abnormalities after exposure to a range of chemical mutagens and carcinogens, fluorouracil did not produce any abnormalities at oral doses of up to 80 mg/kg/day. In female rats, fluorouracil, administered intraperitoneally at weekly doses of 25 or 50 mg/kg for three weeks during the pre-ovulatory phases of oogenesis, significantly reduced the incidence of fertile matings, delayed the development of pre- and post-implantation embryos, increased the incidence of pre-implantation lethality and induced chromosomal anomalies in these embryos. In a limited study in rabbits, a single 25 mg/kg dose of fluorouracil or 5 daily doses of 5 mg/kg had no effect on ovulation, appeared not to affect implantation and had only a limited effect in producing zygote destruction. Compounds such as fluorouracil, which interfere with DNA, RNA and protein synthesis, might be expected to have adverse effects on gametogenesis.
Clinical Studies
There is limited information regarding Clinical Studies of Fluorouracil in the drug label.
How Supplied
- Fluorouracil ® Injection (fluorouracil Injection, USP) is available in two pharmacy bulk vials as follows:

- The 50 mL and 100 mL pharmacy bulk packages are packaged 5 vials per shelf pack.
Storage
- Store at room temperature 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light. Retain in carton until time of use.
NOTE: Although fluorouracil solution may discolor slightly during storage, the potency and safety are not adversely affected.
- Also available as follows:

Images
Drug Images
{{#ask: Page Name::Fluorouracil (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fluorouracil (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed of expected toxic effects, particularly oral manifestations. Patients should be alerted to the possibility of alopecia as a result of therapy and should be informed that it is usually a transient effect.
Precautions with Alcohol
- Alcohol-Fluorouracil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ADRUCIL®
Look-Alike Drug Names
There is limited information regarding Fluorouracil (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Fluorouracil (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Fluorouracil (injection) |Label Name=Fluorouracil05.png
}}
{{#subobject:
|Label Page=Fluorouracil (injection) |Label Name=Fluorouracil06.png
}}
{{#subobject:
|Label Page=Fluorouracil (injection) |Label Name=Fluorouracil07.png
}}