Familial amyloidosis pathophysiology: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 34: | Line 34: | ||
***Cystatin C is a serine protease inhibitor. | ***Cystatin C is a serine protease inhibitor. | ||
***Leu68Gln mutation in its gene leads to cystatin C amyloidosis. | ***Leu68Gln mutation in its gene leads to cystatin C amyloidosis. | ||
**Fibrinogen Aa-chain amyloidosis (A Fib) | **Fibrinogen Aa-chain amyloidosis (A Fib)<ref name="pmid8113408">{{cite journal |vauthors=Uemichi T, Liepnieks JJ, Benson MD |title=Hereditary renal amyloidosis with a novel variant fibrinogen |journal=J. Clin. Invest. |volume=93 |issue=2 |pages=731–6 |date=February 1994 |pmid=8113408 |pmc=293912 |doi=10.1172/JCI117027 |url=}}</ref> | ||
***4 different mutations including Arg554Leu, Glu526Val, 4904delG, and 4897delT has been found to be associated with amyloidosis. | |||
**Apolipoprotein AII amyloidosis (A ApoAII) | **Apolipoprotein AII amyloidosis (A ApoAII) | ||
Revision as of 16:53, 7 November 2019
|
Familial amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Familial amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Familial amyloidosis pathophysiology |
|
Risk calculators and risk factors for Familial amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief:
Overview
Pathophysiology
Pathogenesis
- It is understood that amyloidosis is the result of deposition of Amyloid.[1]
- Amyloid is an abnormal insoluble extracellular protein which may cause organic dysfunction and a wide variety of clinical syndromes.
- These abnormal amyloids are derived from misfolding and aggregation of normally soluble proteins.
- Amyloid depositions also have glycosaminoglycans and serum amyloid P component (SAP) which alter the propensity for amyloid formation.[2][3][4]
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[5]
- Genetic mutations in different genes may lead to misfolding protein product:
- Transthyretin amyloidosis (ATTR)[6][7][8]
- The most common type of familial amyloidosis.
- Single nucleotide substitution on transthyretin gene on chromosome 18 leads to nonfunctional transthyretin protein.
- Transthyretin protein is responsible for thyroid hormone and vitamin A transport and is produced by liver.
- We can find normal transthyretin protein deposition in aged individuals (senile cardiac amyloid).
- Mutant transthyretin protein accelerate the process of deposition and leads to early onset disease.
- Apolipoprotein AI amyloidosis (A ApoAI)[9]
- Single nucleotide substitutions in apolipoprotein AI gene.
- The underlying pathogenesis is incomplete degradation of this protein in body.
- The mode of inheritance in autosomal dominant with different penetrance.
- Gelsolin amyloidosis (A Gel)[10][11]
- Gelsolin protein is produced in skeletal muscle and macrophages.
- 2 different mutations in gelsolin gene on chromosome 9 including Asp187Asn and Asp187Tyr leads to amyloid deposition and Gelsolin amyloidosis.
- Lysozyme amyloidosis (A Lys)[12]
- 4 different mutations on lysozyme gene including Ile56Thr, Asp67His, Trp64Arg, and Phe57Ile has been found to be associated with amyloidosis.
- Cystatin C amyloidosis (A Cys)[13][14]
- Cystatin C is a serine protease inhibitor.
- Leu68Gln mutation in its gene leads to cystatin C amyloidosis.
- Fibrinogen Aa-chain amyloidosis (A Fib)[15]
- 4 different mutations including Arg554Leu, Glu526Val, 4904delG, and 4897delT has been found to be associated with amyloidosis.
- Apolipoprotein AII amyloidosis (A ApoAII)
- Transthyretin amyloidosis (ATTR)[6][7][8]
Genetics
- Familial ATTR amyloidosis is transmitted in autosomal dominant pattern but it can have a heterogeneous nature of presentation.[16][17][18]
- Genes involved in the pathogenesis of Familial ATTR amyloidosis include:
Associated Conditions
Conditions associated with amyloidosis include:[19]
- MEN2A
Gross Pathology
On gross pathology, the organs affected by amyloidosis can be characterized by the following features:
- Porcelain like or waxy appearance
- Enlargement
Images
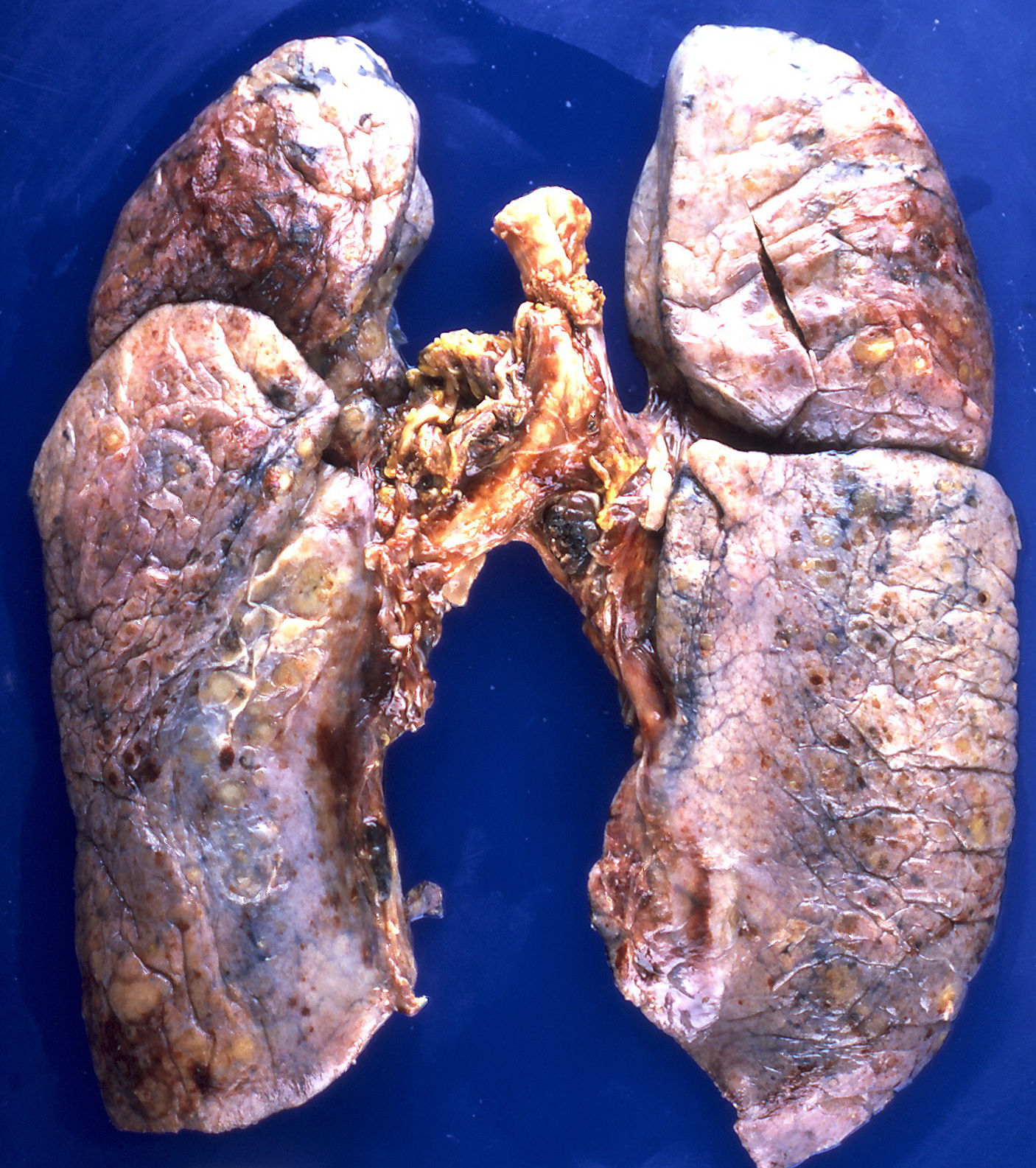
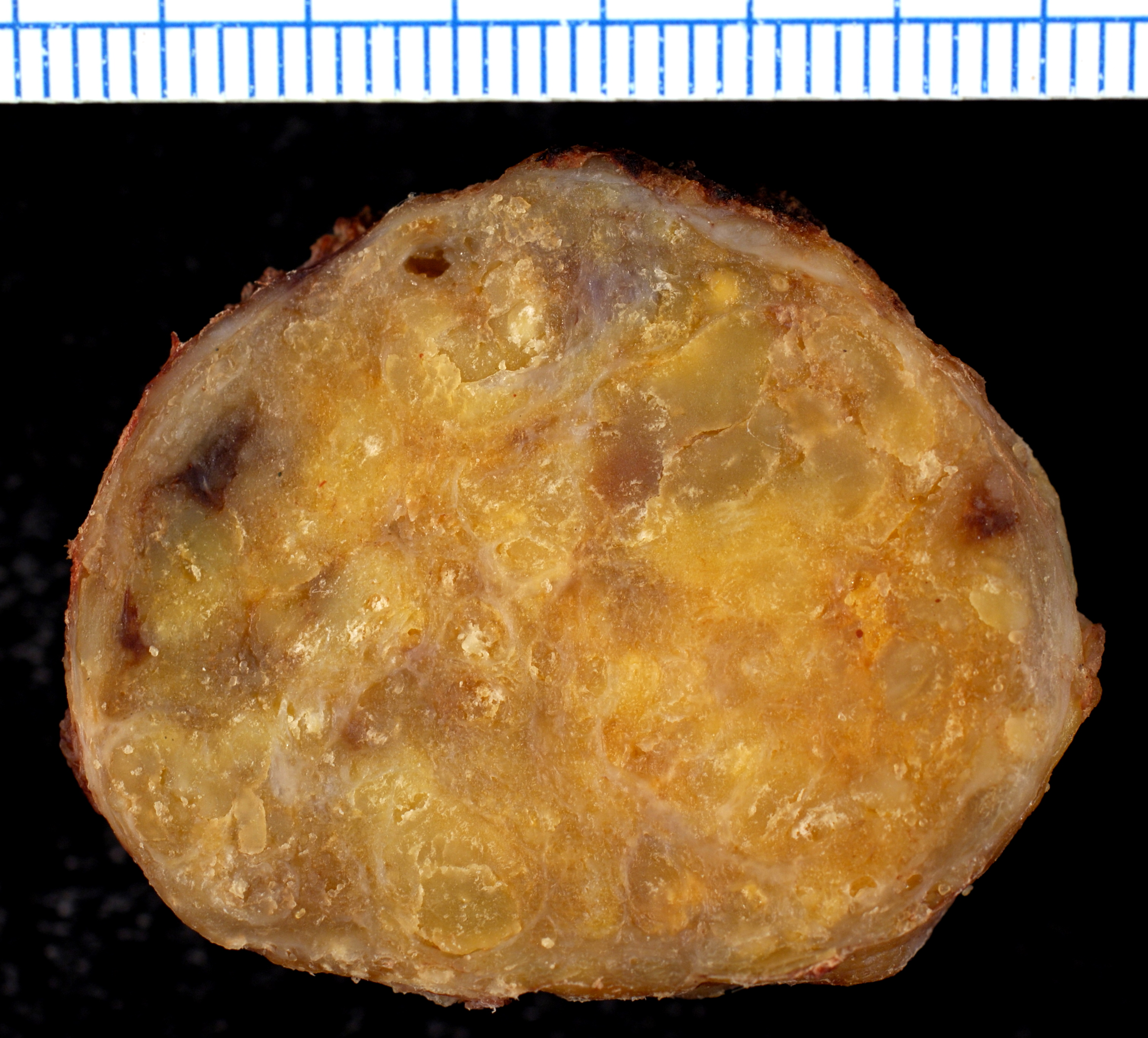

Microscopic Pathology
On microscopic histopathological analysis, amyloidosis is characterized by:[18][23]
- Green birefringence under polarized light after Congo red staining (appears red under normal light)
- Linear non-branching fibrils (indefinite length with an approximately same diameter)
- Distinct X-ray diffraction pattern consistent with Pauling's model of a cross-beta fibril
Images



References
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Pepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Noble GE, Hutchinson WL, Hawkins PN, Nelson SR, Gallimore JR, Herbert J (June 1994). "Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure". Proc. Natl. Acad. Sci. U.S.A. 91 (12): 5602–6. doi:10.1073/pnas.91.12.5602. PMC 44044. PMID 8202534.
- ↑ Tan SY, Pepys MB (November 1994). "Amyloidosis". Histopathology. 25 (5): 403–14. doi:10.1111/j.1365-2559.1994.tb00001.x. PMID 7868080.
- ↑ Botto M, Hawkins PN, Bickerstaff MC, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB (August 1997). "Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene". Nat. Med. 3 (8): 855–9. doi:10.1038/nm0897-855. PMID 9256275.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Robbins J (1976). "Thyroxine-binding proteins". Prog. Clin. Biol. Res. 5: 331–55. PMID 61594.
- ↑ Westermark P, Sletten K, Johansson B, Cornwell GG (April 1990). "Fibril in senile systemic amyloidosis is derived from normal transthyretin". Proc. Natl. Acad. Sci. U.S.A. 87 (7): 2843–5. doi:10.1073/pnas.87.7.2843. PMC 53787. PMID 2320592.
- ↑ Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, Andersen O, Karlberg I, Nordén G, Nakazato M (September 1991). "Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30)". Clin. Genet. 40 (3): 242–6. doi:10.1111/j.1399-0004.1991.tb03085.x. PMID 1685359.
- ↑ Borhani DW, Rogers DP, Engler JA, Brouillette CG (November 1997). "Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation". Proc. Natl. Acad. Sci. U.S.A. 94 (23): 12291–6. doi:10.1073/pnas.94.23.12291. PMC 24911. PMID 9356442.
- ↑ Maury CP, Kere J, Tolvanen R, de la Chapelle A (December 1990). "Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene". FEBS Lett. 276 (1–2): 75–7. doi:10.1016/0014-5793(90)80510-p. PMID 2176164.
- ↑ de la Chapelle A, Tolvanen R, Boysen G, Santavy J, Bleeker-Wagemakers L, Maury CP, Kere J (October 1992). "Gelsolin-derived familial amyloidosis caused by asparagine or tyrosine substitution for aspartic acid at residue 187". Nat. Genet. 2 (2): 157–60. doi:10.1038/ng1092-157. PMID 1338910.
- ↑ Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ (April 1993). "Human lysozyme gene mutations cause hereditary systemic amyloidosis". Nature. 362 (6420): 553–7. doi:10.1038/362553a0. PMID 8464497.
- ↑ Gudmundsson G, Hallgrímsson J, Jónasson TA, Bjarnason O (1972). "Hereditary cerebral haemorrhage with amyloidosis". Brain. 95 (2): 387–404. doi:10.1093/brain/95.2.387. PMID 4655034.
- ↑ Ghiso J, Pons-Estel B, Frangione B (April 1986). "Hereditary cerebral amyloid angiopathy: the amyloid fibrils contain a protein which is a variant of cystatin C, an inhibitor of lysosomal cysteine proteases". Biochem. Biophys. Res. Commun. 136 (2): 548–54. doi:10.1016/0006-291x(86)90475-4. PMID 3707586.
- ↑ Uemichi T, Liepnieks JJ, Benson MD (February 1994). "Hereditary renal amyloidosis with a novel variant fibrinogen". J. Clin. Invest. 93 (2): 731–6. doi:10.1172/JCI117027. PMC 293912. PMID 8113408.
- ↑ Hund E, Linke RP, Willig F, Grau A (February 2001). "Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment". Neurology. 56 (4): 431–5. doi:10.1212/wnl.56.4.431. PMID 11261421.
- ↑ Gertz MA (June 2017). "Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges". Am J Manag Care. 23 (7 Suppl): S107–S112. PMID 28978215.
- ↑ 18.0 18.1
- ↑ Hofstra RM, Sijmons RH, Stelwagen T, Stulp RP, Kousseff BG, Lips CJ, Steijlen PM, Van Voorst Vader PC, Buys CH (August 1996). "RET mutation screening in familial cutaneous lichen amyloidosis and in skin amyloidosis associated with multiple endocrine neoplasia". J. Invest. Dermatol. 107 (2): 215–8. doi:10.1111/1523-1747.ep12329651. PMID 8757765.
- ↑ By Yale Rosen from USA - Amyloidosis, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=31127928
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377537238/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629764
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377538012/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629740
- ↑ Röcken C, Shakespeare A (February 2002). "Pathology, diagnosis and pathogenesis of AA amyloidosis". Virchows Arch. 440 (2): 111–122. doi:10.1007/s00428-001-0582-9. PMID 11964039.
- ↑ By Michael Feldman, MD, PhDUniversity of Pennsylvania School of Medicine - http://www.healcentral.org/healapp/showMetadata?metadataId=38717, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=870218
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559787/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629716
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559955/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629705