Endophthalmitis
|
WikiDoc Resources for Endophthalmitis |
|
Articles |
|---|
|
Most recent articles on Endophthalmitis Most cited articles on Endophthalmitis |
|
Media |
|
Powerpoint slides on Endophthalmitis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Endophthalmitis at Clinical Trials.gov Trial results on Endophthalmitis Clinical Trials on Endophthalmitis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Endophthalmitis NICE Guidance on Endophthalmitis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Endophthalmitis Discussion groups on Endophthalmitis Patient Handouts on Endophthalmitis Directions to Hospitals Treating Endophthalmitis Risk calculators and risk factors for Endophthalmitis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Endophthalmitis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Enophthalmitis may be classified according to causative organisms into 2 subtypes: Bacterial and fungal.
Additionally, endophthalmitis is classified as either endogenous or exogenous based on the route of infection.
- Exogenous endophthalmitis
- Endogenous endophthalmitis
Overview
Endophthalmitis is an inflammation of the internal coats of the eye. It is a dreaded complication of all intraocular surgeries, particularly cataract surgery, with possible loss of vision and the eye itself. Infectious etiology is the most common and various bacteria and fungi have been isolated as the cause of the endophthalmitis. Other causes include penetrating trauma and retained intraocular foreign bodies.
Pathophysiology
Pathogenesis
Endophthalmitis is an ocular inflammation resulting from the introduction of an infectious agent, either bacterial or fungal, into the posterior segment of the eye. Infectious agents are introduce to the anterior and posterior segments of the eye exogenously or endogenously.[1][2]
Exogenous enophthalmitis most commonly occurs following cataract surgery (approximately 90% of all cases) Beside cataract, nearly all other type of ocular surgery, such as glaucoma, retinal, radial keratotomy, and intravitreal injections, may be able to disturb the eye globe integrity and contaminate the aqueous humor and/or vitreous.[1][2][3] Most commonly endophthalmitis occurs following penetrating ocular injuries. Following penetrating injury, the eye globe integrity disturbed. Penetrating ocular injuries are accompanied by infection at a much higher rate compere to those with ocular surgery. It is also associated with a greater variety of organisms. The most common isolated organisms include Gram-positive Staphylococcus epidermidis and Streptococcus.[2][4]
Endogenous endophthalmitis is caused by the hematologic dissemination of an infection to the eyes. Most common extraocular foci of infection include liver abscess, pneumonia, endocarditis, and soft tissue infection. Endogenous endophthalmitis is commonly associated with immunosuppression or procedures that increase the risk for blood-borne infections, such as diabetes, HIV, malignancy, intravenous drug use, transplantation, immunosuppressive therapy, and catheterization.[1][2][5]
Gross Pathology
On gross pathology, eyelid swelling, eyelid erythema, injected conjunctiva and sclera, hypopyon, chemosis, and mucoprulunt dischage are characteristic findings of exogenous bacterial endophthalmitis.
Microscopic histopathological analysis
On microscopic histopathological analysis, infiltration of polymorphonuclear leukocytes or chronic inflammatory cells (depending on the duration of the inflammation) and destruction of ocular structures are characteristic findings of bacterial endophthalmitis.
Causes
Drug side-effect
Infectious
Common bacterial and fungal causes of endophthalmitis include:
- Bacterial
- Fungal
Epidemiology and Demographics
- In the United States, post-cataract endophthalmitis is the most common form of endophthalmitis.
- The incidence of post-cataract endophthalmitis was estimated to range from 80 to 360 cases per 100,00 individuals with ocular surgery.[6]
Risk Factors
Endogenous bacterial endophthalmitis
Common risk factors in the development of endogenous bacterial endophthalmitis include:[1][2][5]
- Recent hospitalization
- Immunosuppression
- Diabetes mellitus
Signs and symptoms
A history of recent intraocular surgery or penetrating ocular trauma is usually elicited. In some cases of metastatic endophthalmitis, the spread of infection may be hematogenous (via the blood-stream). That is more commonly seen in patients with immunocompromised states like AIDS and also in diabetes. The condition is usually accompanied by severe pain, loss of vision and redness of the conjunctiva and the underlying episclera. Alongside are present signs of inflammation of the various coats of the eye. Hypopyon can also be present in endophthalmitis and should be looked for on examination by a slit lamp. Progression to involve all the coats of the eye is called as panuveitis or panophthalmitis.
Slit lamp finding
- Hypopyon ( >80% of cases)
- Anterior chamber and vitreous inflammation
- Cloudy cornea
- Clumps of exudate in the anterior chamber (around the pupillary margin)
- Cloudy cornea
- Decreased red reflex
-
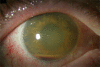
Anterior chamber inflammation, mild corneal edema, and hypopyon in bacterial endophthalmitis
-
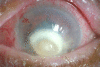
Exogenous fungal endophthalmitis with corneal ulcer
-
(A) Severe conjunctival injection, subconjunctival hemorrhage, corneal stromal edema, and hypopyon (B) Fundus photograph shows a mild pale color of optic disc & macular degeneration[7]
-
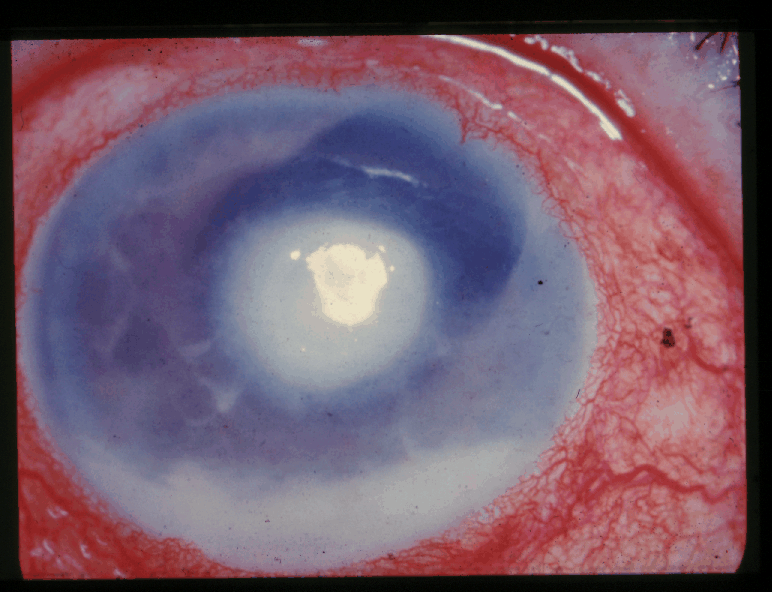
Corneal edema and severe anterior chamber exudation[8]
Treatment
- The patient needs urgent examination by an expert ophthalmologist and/or vitreo-retina specialist who will usually decide for urgent intervention to provide intravitreal injection of potent antibiotics and also prepare for an urgent pars plana vitrectomy as needed. Enucleation may be required to remove a blind and painful eye.
- Endogenous endophthalmitis is caused by the hematologic dissemination of an infection to the eyes. Systemic antibiotics are recommended in endogenous bacterial endophthalmitis because the source of the infection is distant from the eye.
- Bacterial and fungal cultures from vitreous samples are necessary in the management of endophthalmitis
- Immediate vitrectomy is often necessary
Antimicrobial Regimens
- Infectious endophthalmitis[1]
- 1. Causative pathogens
- 2. Empiric antimicrobial therapy
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Vancomycin 1 g IV bid for 2 weeks AND Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 1 g IV bid for 2 weeks AND Clindamycin 600-1200 mg IV bid to qid for 2 weeks
- Note (1): Re-injection should be considered if the infection does not improve beyond 48 hours of the first injection. Re-injection significantly increases the risk of retinal toxicity.
- Note (2): In addition to intravitreal and systemic antibiotic therapy, vitrectomy is usually necessary
- Note (3): Intravitreal and intravenous Amphotericin B may be added to the regimen if fungal endophthalmitis is suspected
- 3. Pathogen-directed antimicrobial therapy
- 3.1 Bacillus spp.
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Vancomycin 1 g IV bid for 2 weeks AND Clindamycin 600-1200 mg IV bid to qid for 2 weeks
- Note: In addition to antimicrobial therapy, vitrectomy is usually necessary
- 3.2 Non-Bacillus gram-positive bacteria
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Vancomycin 1 g IV bid for 2 weeks
- Note: In addition to antimicrobial therapy, vitrectomy is usually necessary
- 3.3 Gram-negative bacteria
- Preferred regimen: Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 1 g IV bid for 2 weeks
- Note: In addition to antimicrobial therapy, vitrectomy is usually necessary
- 3.4 Candida spp.
- Preferred regimen: (Fluconazole 400-800 mg IV/PO qd for 6-12 weeks OR Voriconazole 400 mg IV/PO bid for 2 doses followed by 200-300 mg IV/PO bid for 6-12 weeks OR Amphotericin B 0.7-1.0 mg/kg IV qd for 6-12 weeks) AND Amphotericin B 5-10 microgram in 0.1 mL in normal saline intravitreal injection, single dose
- Note (1): In addition to antimicrobial therapy, vitrectomy is usually necessary
- 3.5 Aspergillus spp.
- Preferred regimen: Amphotericin B 5-10 microgram in 0.1 mL normal saline intravitreal injection, single dose AND Dexamethasone 400 microgram intravitreal injection, single dose
- Note (1): In addition to antimicrobial therapy, vitrectomy is usually necessary
- Note (2): Repeat antimicrobial regimen in 2 days post-vitrectomy
- 4. Special Considerations
- 4.1 Endogenous endophthalmitis
- 4.1.1 Empiric antimicrobial therapy
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Vancomycin 1 g IV bid for 2 weeks AND Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 1 g IV bid for 2 weeks AND Clindamycin 600-1200 mg IV bid to qid for 2 weeks
- Note (1): Re-injection should be considered if the infection does not improve beyond 48 hours of the first injection. Re-injection significantly increases the risk of retinal toxicity.
- Note (2): In addition to intravitreal and systemic antibiotic therapy, vitrectomy is usually necessary::* Note (3): Intravitreal and intravenous Amphotericin B may be added to the regimen if fungal endophthalmitis is suspected
- 4.2 Bleb-related endophthalmitis
- 4.2.1 Empiric antimicrobial therapy
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Vancomycin 1 g IV bid for 2 weeks AND Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 1 g IV bid for 2 weeks AND Clindamycin 600-1200 mg IV bid to qid for 2 weeks
- Note (1): Re-injection should be considered if the infection does not improve beyond 48 hours of the first injection. Re-injection significantly increases the risk of retinal toxicity.
- Note (2): In addition to intravitreal and systemic antibiotic therapy, vitrectomy is usually necessary
- 4.3 Post-operative endophthalmitis
- 4.3.1 Empiric antimicrobial therapy
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose
- Note (1): In addition to intravitreal antibiotic therapy, vitrectomy is necessary
- Note (2): If there is no improvement in 48 h, a repeat intravitreal injection may be administered
- Note (3): Late post-operative endophthalmitis is often caused by Propionibacterium acnes (several years post-op)
- 4.3.2 Pathogen-directed antimicrobial therapy
- 4.3.2.1 Gram-positive bacteria
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose
- 4.3.2.2 Gram-negative bacteria
- Preferred regimen: Amikacin 0.4 mg per 0.1 mL normal saline intravitreal injection, single dose
- Note: Intravitreal amikacin is associated with the development of retinal microvasculitis
- 4.4 Post-traumatic endophthalmitis
- 4.4.1 Empiric antimicrobial therapy
- Preferred regimen: Vancomycin 1 mg per 0.1 mL normal saline intravitreal injection, single dose AND Ceftazidime 2.25 mg per 0.1 mL normal saline intravitreal injection, single dose AND Amphotericin B 5-10 microgram in 0.1 mL in normal saline intravitreal injection, single dose
- Note (1): Removal of foreign bodies and debridement of necrotic tissue is necessary
- Note (2): In addition to antimicrobial therapy, vitrectomy is necessary
- Note (3): Systemic broad spectrum antibiotics are recommended in post-traumatic endophthalmitis
External links
- Peters JR. "Endophthalmitis." eMedicine.com. Accessed September 28, 2006.
- Wu L. "Endophthalmitis, Fungal." eMedicine.com. Accessed September 28, 2006.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Durand ML (2013). "Endophthalmitis". Clin Microbiol Infect. 19 (3): 227–34. doi:10.1111/1469-0691.12118. PMC 3638360. PMID 23438028.
- ↑ 2.0 2.1 2.2 2.3 2.4 Kernt M, Kampik A (2010). "Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives". Clin Ophthalmol. 4: 121–35. PMC 2850824. PMID 20390032.
- ↑ Keay L, Gower EW, Cassard SD, Tielsch JM, Schein OD (2012). "Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries". Ophthalmology. 119 (5): 914–22. doi:10.1016/j.ophtha.2011.11.023. PMC 3343208. PMID 22297029.
- ↑ Essex RW, Yi Q, Charles PG, Allen PJ (2004). "Post-traumatic endophthalmitis". Ophthalmology. 111 (11): 2015–22. doi:10.1016/j.ophtha.2003.09.041. PMID 15522366.
- ↑ 5.0 5.1 Wong JS, Chan TK, Lee HM, Chee SP (2000). "Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction". Ophthalmology. 107 (8): 1483–91. PMID 10919895.
- ↑ Aaberg TM, Flynn HW, Schiffman J, Newton J (1998). "Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes". Ophthalmology. 105 (6): 1004–10. doi:10.1016/S0161-6420(98)96000-6. PMID 9627649.
- ↑ Seo SW, Chung IY, Kim E, Park JM (2008). "A case of postoperative Sphingomonas paucimobilis endophthalmitis after cataract extraction". Korean J Ophthalmol. 22 (1): 63–5. doi:10.3341/kjo.2008.22.1.63. PMC 2629956. PMID 18323709.
- ↑ Jaru-Ampornpan P, Agarwal A, Midha NK, Kim SJ (2011). "Traumatic Endophthalmitis due to Cellulosimicrobium cellulans". Case Rep Ophthalmol Med. 2011: 469607. doi:10.1155/2011/469607. PMC 3350247. PMID 22606461.
![Corneal edema and severe anterior chamber exudation[8]](/images/c/c4/IndianJOphthalmol-55-464-g001.gif)