Dupilumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand, Anmol Pitliya, M.B.B.S. M.D.[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dupilumab is a human monoclonal IgG4 antibody that is FDA approved for the treatment of moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Common adverse reactions include injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, and dry eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Dupilumab is indicated for the treatment of adult patients with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Dupilumab can be used with or without topical corticosteroids.
Dosage
- Dupilumab is administered by subcutaneous injection.
- The recommended dose of dupilumab for adult patients is an initial dose of 600 mg (two 300 mg injections), followed by 300 mg given every other week.
- Dupilumab can be used with or without topical corticosteroids. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas.
- If a dose is missed, instruct the patient to administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, instruct the patient to wait until the next dose on the original schedule.
Dosage Forms and Strengths
- Dupilumab is a clear to slightly opalescent, colorless to pale yellow solution available as:
- Injection: 300 mg/2 mL in a single-dose pre-filled syringe with needle shield
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding dupilumab Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding dupilumab Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Dupilumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding dupilumab Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding dupilumab Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Dupilumab is contraindicated in patients who have known hypersensitivity to dupilumab or any of its excipients.
Warnings
Hypersensitivity
- Hypersensitivity reactions, including generalized urticaria and serum sickness or serum sickness-like reactions, were reported in less than 1% of subjects who received dupilumab in clinical trials. Two subjects experienced serum sickness or serum sickness-like reactions that were associated with high titers of antibodies to dupilumab. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue dupilumab.
Conjunctivitis and Keratitis
- Conjunctivitis and keratitis occurred more frequently in subjects who received dupilumab. Conjunctivitis was the most frequently reported eye disorder. Most subjects with conjunctivitis recovered or were recovering during the treatment period.
- Keratitis was reported in <1% of the dupilumab group (1 per 100 subject-years) and in 0% of the placebo group (0 per 100 subject-years) in the 16-week monotherapy trials. In the 52-week dupilumab + topical corticosteroids (TCS) trial, keratitis was reported in 4% of the dupilumab + TCS group (12 per 100 subject-years) and in 0% of the placebo + TCS group (0 per 100 subject-years). Most subjects with keratitis recovered or were recovering during the treatment period.
- Advise patients to report new onset or worsening eye symptoms to their healthcare provider.
Comorbid Asthma
- Safety and efficacy of dupilumab have not been established in the treatment of asthma. Advise patients with comorbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Parasitic (Helminth) Infections
- Patients with known helminth infections were excluded from participation in clinical studies. It is unknown if dupilumab will influence the immune response against helminth infections.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Three randomized, double-blind, placebo-controlled, multicenter trials (Trials 1, 2, and 3) and one dose-ranging trial (Trial 4) evaluated the safety of dupilumab in subjects with moderate-to-severe atopic dermatitis. The safety population had a mean age of 38 years; 41% of subjects were female, 67% were white, 24% were Asian, and 6% were black; in terms of comorbid conditions, 48% of the subjects had asthma, 49% had allergic rhinitis, 37% had food allergy, and 27% had allergic conjunctivitis. In these 4 trials, 1472 subjects were treated with subcutaneous injections of dupilumab, with or without concomitant topical corticosteroids (TCS).
- A total of 739 subjects were treated with dupilumab for at least 1 year in the development program for moderate-to-severe atopic dermatitis.
- Trials 1, 2, and 4 compared the safety of dupilumab monotherapy to placebo through Week 16. Trial 3 compared the safety of dupilumab + TCS to placebo + TCS through Week 52.
Weeks 0 to 16 (Trials 1 to 4):
- In dupilumab monotherapy trials (Trials 1, 2, and 4) through Week 16, the proportion of subjects who discontinued treatment because of adverse events was 1.9% in both the dupilumab 300 mg Q2W and placebo groups.
- Table 1 summarizes the adverse reactions that occurred at a rate of at least 1% in the dupilumab 300 mg Q2W monotherapy groups, and in the dupilumab + TCS group, all at a higher rate than in their respective comparator groups during the first 16 weeks of treatment.
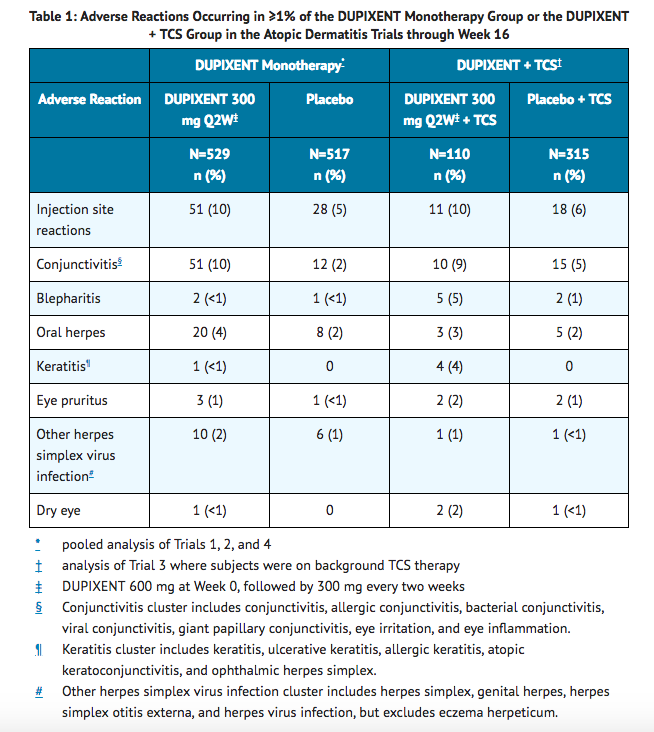
Safety through Week 52 (Trial 3):
- In the dupilumab with concomitant TCS trial (Trial 3) through Week 52, the proportion of subjects who discontinued treatment because of adverse events was 1.8% in dupilumab 300 mg Q2W + TCS group and 7.6% in the placebo + TCS group. Two subjects discontinued dupilumab because of adverse reactions: atopic dermatitis (1 subject) and exfoliative dermatitis (1 subject).
- The safety profile of dupilumab + TCS through Week 52 was generally consistent with the safety profile observed at Week 16.
Specific Adverse Reactions
Conjunctivitis
- During the 52-week treatment period of concomitant therapy trial (Trial 3), conjunctivitis was reported in 16% of the dupilumab 300 mg Q2W + TCS group (20 per 100 subject-years) and in 9% of the placebo + TCS group (10 per 100 subject-years).
Eczema Herpeticum and Herpes Zoster
- The rate of eczema herpeticum was similar in the placebo and dupilumab groups.
- Herpes zoster was reported in <0.1% of the dupilumab groups (<1 per 100 subject-years) and in <1% of the placebo group (1 per 100 subject-years) in the 16-week monotherapy trials. In the 52-week dupilumab + TCS trial, herpes zoster was reported in 1% of the dupilumab + TCS group (1 per 100 subject-years) and 2% of the placebo + TCS group (2 per 100 subject-years).
Hypersensitivity Reactions
- Hypersensitivity reactions were reported in <1% of dupilumab-treated subjects. These included serum sickness reaction, serum sickness-like reaction, and generalized urticaria.
Eosinophils
- Dupilumab-treated subjects had a greater mean initial increase from baseline in eosinophil count compared to subjects treated with placebo in the monotherapy trials. Eosinophil counts declined to near baseline levels by Week 16. The initial increase in eosinophils was not observed in the 52-week dupilumab + TCS trial.
- In Trials 1, 2, and 3, the incidence of treatment-emergent eosinophilia (≥500 cells/mcL) was similar in dupilumab and placebo groups. In Trials 1, 2, and 3, treatment-emergent eosinophilia (≥5,000 cells/mcL) was reported in <1% of dupilumab-treated patients and none in placebo-treated patients. In most cases, eosinophil counts declined to near baseline during study treatment.
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to dupilumab in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
- Approximately 7% of subjects with atopic dermatitis who received dupilumab 300 mg Q2W for 16 weeks developed antibodies to dupilumab. Of the subjects who developed antibodies to dupilumab, approximately 30% (2% of all subjects receiving dupilumab) had antibodies that were classified as neutralizing.
- Of the subjects with atopic dermatitis who received dupilumab 300 mg Q2W + TCS for 52 weeks, approximately 7% developed antibodies to dupilumab and approximately 2% had persistent antibody responses, defined as having at least 2 consecutive positive post-baseline samples. Of the subjects who developed antibodies to dupilumab, approximately 14% (1% of all subjects receiving dupilumab + TCS) had antibodies that were classified as neutralizing.
- In subjects who received dupilumab, development of antibodies to dupilumab was associated with lower serum dupilumab concentrations.
- Antibodies to dupilumab were detected in approximately 2% and 8% of subjects with atopic dermatitis in the placebo or the placebo + TCS groups, respectively.
- The antibody titers detected in both dupilumab and placebo subjects were generally low. Two subjects developed serum sickness or serum sickness-like reactions and high titers of antibodies to dupilumab during dupilumab therapy.
Postmarketing Experience
There is limited information regarding Dupilumab Postmarketing Experience in the drug label.
Drug Interactions
- Live Vaccines
- Non-Live Vaccines
Live Vaccines
- Avoid use of live vaccines in patients treated with dupilumab.
Non-Live Vaccines
- Immune responses to vaccination were assessed in a study in which subjects with atopic dermatitis were treated once weekly for 16 weeks with 300 mg of dupilumab (twice the recommended dosing frequency). After 12 weeks of dupilumab administration, subjects were vaccinated with a Tdap vaccine (Adacel®) and a meningococcal polysaccharide vaccine (Menomune®). Antibody responses to tetanus toxoid and serogroup C meningococcal polysaccharide were assessed 4 weeks later. Antibody responses to both tetanus vaccine and meningococcal polysaccharide vaccine were similar in dupilumab-treated and placebo-treated subjects. Immune responses to the other active components of the Adacel and Menomune vaccines were not assessed.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no available data on dupilumab use in pregnant women to inform any drug associated risk. Human IgG antibodies are known to cross the placental barrier; therefore, dupilumab may be transmitted from the mother to the developing fetus. In an enhanced pre- and post-natal developmental study, no adverse developmental effects were observed in offspring born to pregnant monkeys after subcutaneous administration of a homologous antibody against interleukin-4-receptor alpha (IL-4Rα) during organogenesis through parturition at doses up to 10-times the maximum recommended human dose (MRHD) [see DATA]. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data (Animal)
- In an enhanced pre- and post-natal development toxicity study, pregnant cynomolgus monkeys were administered weekly subcutaneous doses of homologous antibody against IL-4Rα up to 10 times the MRHD (on a mg/kg basis of 100 mg/kg/week) from the beginning of organogenesis to parturition. No treatment-related adverse effects on embryofetal toxicity or malformations, or on morphological, functional, or immunological development were observed in the infants from birth through 6 months of age.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dupilumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dupilumab during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of dupilumab in human milk, the effects on the breastfed infant, or the effects on milk production. Human IgG is known to be present in human milk. The effects of local gastrointestinal and limited systemic exposure to dupilumab on the breastfed infant are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for dupilumab and any potential adverse effects on the breastfed child from dupilumab or from the underlying maternal condition.
Pediatric Use
- Safety and efficacy in pediatric patients (<18 years of age) have not been established.
Geriatic Use
- Of the 1472 subjects with atopic dermatitis exposed to dupilumab in a dose-ranging study and placebo-controlled trials, 67 subjects were 65 years or older. Although no differences in safety or efficacy were observed between older and younger subjects, the number of subjects aged 65 and over is not sufficient to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Dupilumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dupilumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dupilumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dupilumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dupilumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dupilumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Important Administration Instructions
- Dupilumab is intended for use under the guidance of a healthcare provider. A patient may self-inject dupilumab after training in subcutaneous injection technique using the pre-filled syringe. Provide proper training to patients and/or caregivers on the preparation and administration of dupilumab prior to use according to the "Instructions for Use".
- For the initial 600 mg dose, administer each of the two dupilumab 300 mg injections at different injection sites.
- Administer subcutaneous injection into the thigh or abdomen, except for the 2 inches (5 cm) around the navel. The upper arm can also be used if a caregiver administers the injection.
- Rotate the injection site with each injection. DO NOT inject dupilumab into skin that is tender, damaged, bruised, or scarred.
- The dupilumab "Instructions for Use" contains more detailed instructions on the preparation and administration of dupilumab
Preparation for Use of dupilumab Pre-filled Syringe With Needle Shield
- Before injection, remove dupilumab pre-filled syringe from the refrigerator and allow dupilumab to reach room temperature (45 minutes) without removing the needle cap.
- Inspect dupilumab visually for particulate matter and discoloration prior to administration. dupilumab is a clear to slightly opalescent, colorless to pale yellow solution. Do not use if the liquid contains visible particulate matter, is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow). Dupilumab does not contain preservatives; therefore, discard any unused product remaining in the pre-filled syringe.
Monitoring
- Improvement in disease severity and reduction in pruritus indicates efficacy.
IV Compatibility
There is limited information regarding the compatibility of Dupilumab and IV administrations.
Overdosage
- There is no specific treatment for dupilumab overdose. In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and institute appropriate symptomatic treatment immediately.
Pharmacology
Mechanism of Action
- Dupilumab is a human monoclonal IgG4 antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling by specifically binding to the IL-4Rα subunit shared by the IL-4 and IL-13 receptor complexes. Dupilumab inhibits IL-4 signaling via the Type I receptor and both IL-4 and IL-13 signaling through the Type II receptor.
- Blocking IL-4Rα with dupilumab inhibits IL-4 and IL-13 cytokine-induced responses, including the release of proinflammatory cytokines, chemokines and IgE.
Structure
There is limited information regarding Dupilumab Structure in the drug label.
Pharmacodynamics
- Consistent with receptor blockade, serum levels of IL-4 and IL-13 were increased following dupilumab treatment. The relationship between the pharmacodynamic activity and the mechanism(s) by which dupilumab exerts its clinical effects is unknown.
Pharmacokinetics
Absorption
- Following an initial subcutaneous (SC) dose of 600 mg, dupilumab reached peak mean ±SD concentrations (Cmax) of 70.1±24.1 mcg/mL by approximately 1 week post dose.
- Steady-state concentrations were achieved by Week 16 following the administration of 600 mg starting dose and 300 mg dose either weekly (twice the recommended dosing frequency) or every other week. Across clinical trials, the mean ±SD steady-state trough concentrations ranged from 73.3±40.0 mcg/mL to 79.9±41.4 mcg/mL for 300 mg administered every 2 weeks and from 173±75.9 mcg/mL to 193±77.0 mcg/mL for 300 mg administered weekly.
- The bioavailability of dupilumab following a SC dose is estimated to be 64%.
Distribution
- The estimated total volume of distribution was approximately 4.8±1.3 L.
Elimination
- The metabolic pathway of dupilumab has not been characterized. As a human monoclonal IgG4 antibody, dupilumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG. After the last steady-state dose of 300 mg Q2W or 300 mg QW dupilumab, the median times to non-detectable concentration (<78 ng/mL) are 10 and 13 weeks, respectively.
Dose Linearity
- Dupilumab exhibited nonlinear target-mediated pharmacokinetics with exposures increasing in a greater than dose-proportional manner. The systemic exposure increased by 30-fold when the dose increased 8-fold following a single dose of dupilumab from 75 mg to 600 mg (i.e., 0.25-times to 2-times the recommended dose).
Weight
- Dupilumab trough concentrations were lower in subjects with higher body weight.
Immunogenicity
- Development of antibodies to dupilumab was associated with lower serum dupilumab concentrations. A few subjects who had high antibody titers also had no detectable serum dupilumab concentrations.
Specific Populations
Geriatric Patients
- In subjects who are 65 years and older, the mean ±SD steady-state trough concentrations of dupilumab were 69.4±31.4 mcg/mL and 166±62.3 mcg/mL, respectively, for 300 mg administered every 2 weeks and weekly. No dose adjustment in this population is recommended.
Renal or Hepatic Impairment
- No formal trial of the effect of hepatic or renal impairment on the pharmacokinetics of dupilumab was conducted.
Drug Interaction Studies
Cytochrome P450 Substrates
- The effects of dupilumab on the pharmacokinetics of midazolam (metabolized by CYP3A4), warfarin (metabolized by CYP2C9), omeprazole (metabolized by CYP2C19), metoprolol (metabolized by CYP2D6), and caffeine (metabolized by CYP1A2) were evaluated in a study with 12-13 evaluable subjects with atopic dermatitis (a SC loading dose of 600 mg followed by 300 mg SC weekly for six weeks). No clinically significant changes in AUC were observed. The largest effect was observed for metoprolol (CYP2D6) with an increase in AUC of 29%.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of dupilumab.
- No effects on fertility parameters such as reproductive organs, menstrual cycle length, or sperm analysis were observed in sexually mature mice that were subcutaneously administered a homologous antibody against IL-4Rα at doses up to 200 mg/kg/week.
Clinical Studies
- Three randomized, double-blind, placebo-controlled trials (Trials 1, 2, and 3) enrolled a total of 2119 subjects 18 years of age and older with moderate-to-severe atopic dermatitis (AD) not adequately controlled by topical medication(s). Disease severity was defined by an Investigator's Global Assessment (IGA) score ≥3 in the overall assessment of AD lesions on a severity scale of 0 to 4, an Eczema Area and Severity Index (EASI) score ≥16 on a scale of 0 to 72, and a minimum body surface area involvement of ≥10%. At baseline, 59% of subjects were male, 67% were white, 52% of subjects had a baseline IGA score of 3 (moderate AD), and 48% of subjects had a baseline IGA of 4 (severe AD). The baseline mean EASI score was 33 and the baseline weekly averaged peak pruritus Numeric Rating Scale (NRS) was 7 on a scale of 0-10.
- In all three trials, subjects in the dupilumab group received subcutaneous injections of dupilumab 600 mg at Week 0, followed by 300 mg every other week (Q2W). In the monotherapy trials (Trials 1 and 2), subjects received dupilumab or placebo for 16 weeks.
- In the concomitant therapy trial (Trial 3), subjects received dupilumab or placebo with concomitant topical corticosteroids (TCS) and as needed topical calcineurin inhibitors for problem areas only, such as the face, neck, intertriginous and genital areas for 52 weeks.
- All three trials assessed the primary endpoint, the change from baseline to Week 16 in the proportion of subjects with an IGA 0 (clear) or 1 (almost clear) and at least a 2-point improvement. Other endpoints included the proportion of subjects with EASI-75 (improvement of at least 75% in EASI score from baseline), and reduction in itch as defined by at least a 4-point improvement in the peak pruritus NRS from baseline to Week 16.
Clinical Response at Week 16 (Trials 1, 2, and 3)
- The results of the DUPIXENT monotherapy trials (Trials 1 and 2) and the DUPIXENT with concomitant TCS trial (Trial 3) are presented in Table 2.
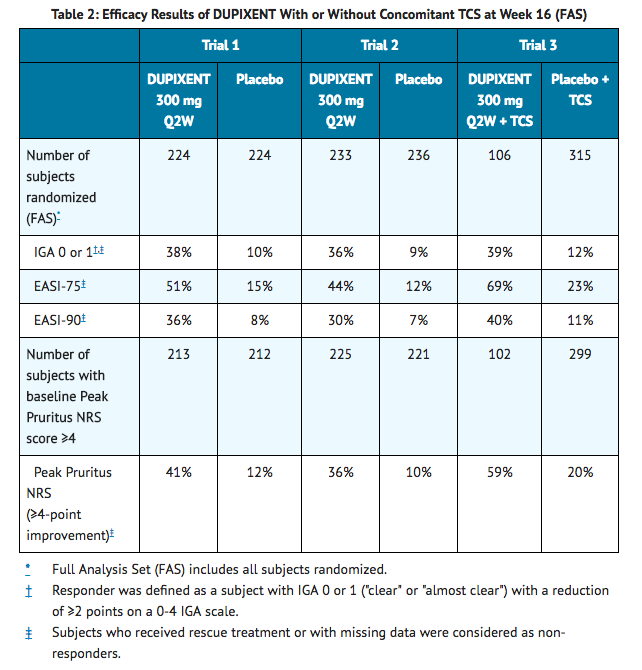
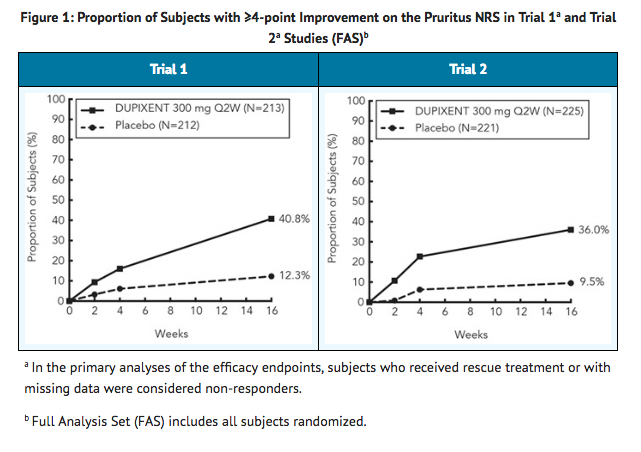
- In Trial 3, of the 421 subjects, 353 had been on study for 52 weeks at the time of data analysis. Of these 353 subjects, responders at Week 52 represent a mixture of subjects who maintained their efficacy from Week 16 (e.g., 53% of dupilumab IGA 0 or 1 responders at Week 16 remained responders at Week 52) and subjects who were non-responders at Week 16 who later responded to treatment (e.g., 24% of dupilumab IGA 0 or 1 non-responders at Week 16 became responders at Week 52). Results of supportive analyses of the 353 subjects in the dupilumab with concomitant TCS trial (Trial 3) are presented in Table 3.
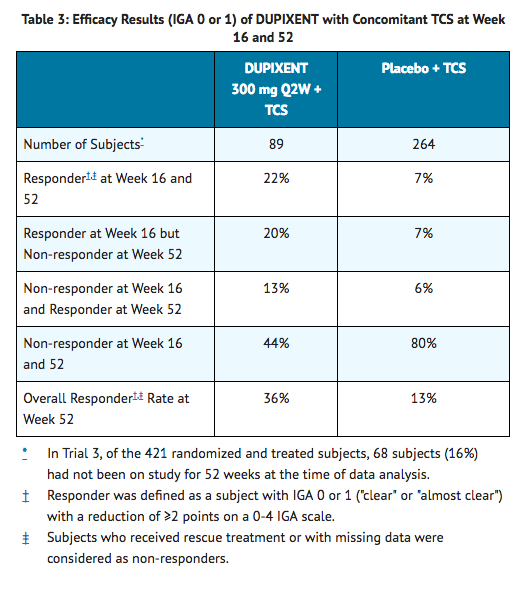
- Treatment effects in subgroups (weight, age, gender, race, and prior treatment, including immunosuppressants) in Trials 1, 2, and 3 were generally consistent with the results in the overall study population.
- In Trials 1, 2, and 3, a third randomized treatment arm of dupilumab 300 mg QW did not demonstrate additional treatment benefit over dupilumab 300 mg Q2W.
- Subjects in Trials 1 and 2 who had an IGA 0 or 1 with a reduction of ≥2 points were re-randomized into Trial 5. Trial 5 evaluated multiple dupilumab monotherapy dose regimens for maintaining treatment response. The study included subjects randomized to continue with dupilumab 300 mg Q2W (62 subjects) or switch to placebo (31 subjects) for 36 weeks. IGA 0 or 1 responses at Week 36 were as follows: 33 (53%) in the Q2W group and 3 (10%) in the placebo group.
How Supplied
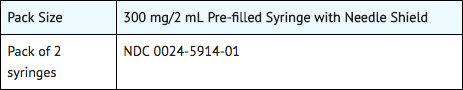
- Dupilumab Injection is a clear to slightly opalescent, colorless to pale yellow solution, supplied in single-dose pre-filled syringes with needle shield. Each pre-filled syringe with needle shield is designed to deliver 300 mg of dupilumab in 2 mL solution.
- Dupilumab is available in cartons containing 2 pre-filled syringes with needle shield.
Storage
- Dupilumab is sterile and preservative-free. Discard any unused portion.
- Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- If necessary, pre-filled syringes may be kept at room temperature up to 77°F (25°C) for a maximum of 14 days. Do not store above 77°F (25°C). After removal from the refrigerator, dupilumab must be used within 14 days or discarded.
- Do not expose the syringe to heat or direct sunlight.
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
- Do NOT freeze. Do NOT expose to heat. Do NOT shake
Images
Drug Images
{{#ask: Page Name::Dupilumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Dupilumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patients and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use) before the patient starts using dupilumab and each time the prescription is renewed as there may be new information they need to know.
Administration Instructions
- Provide proper training to patients and/or caregivers on proper subcutaneous injection technique, including aseptic technique, and the preparation and administration of dupilumab prior to use. Advise patients to follow sharps disposal recommendations.
Hypersensitivity
- Advise patients to discontinue dupilumab and to seek immediate medical attention if they experience any symptoms of systemic hypersensitivity reactions.
Conjunctivitis and Keratitis
- Advise patients to consult their healthcare provider if new onset or worsening eye symptoms develop.
Comorbid Asthma
- Advise patients with comorbid asthma not to adjust or stop their asthma treatment without talking to their physicians.

Precautions with Alcohol
Alcohol-Dupilumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Dupixent
Look-Alike Drug Names
There is limited information regarding Dupilumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.