Dimercaprol: Difference between revisions
No edit summary |
No edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
|authorTag={{SG}} | |authorTag={{SG}} | ||
|genericName=dimercaprol | |genericName=dimercaprol | ||
|aOrAn=a | |||
|drugClass=heavy metal chelator | |drugClass=heavy metal chelator | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=arsenic, gold and mercury poisoning. It is indicated in acute lead poisoning when used concomitantly with Edetate Calcium Disodium Injection USP | |indication=[[arsenic]], [[gold]] and [[mercury poisoning]]. It is indicated in acute [[lead poisoning]] when used concomitantly with [[Edetate Calcium Disodium]] Injection USP | ||
|adverseReactions=Blepharospasm, conjunctivitis, lacrimation, nasal discharge, | |adverseReactions=[[Blepharospasm]], [[conjunctivitis]], [[lacrimation]], [[nasal discharge]], | ||
tightness sensation | tightness sensation in [[chest]], [[limbs]], [[jaw]] and [[abdomen]], injection site pain, [[nausea]], [[vomiting]], [[headache]], [[paresthesia]], [[tremor]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=====Mild Arsenic or Gold Poisoning==== | |||
*2.5 mg/kg of body weight four times daily for two days. | |||
*Two times on the third day. | |||
*Only once daily thereafter for ten days. | |||
====Severe Arsenic or Gold Poisoning==== | |||
*3 mg/kg every four hours for two-days | |||
*Four times on the third day | |||
*Twice daily thereafter for ten days. | |||
====Mercury poisoning==== | |||
*5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days. | |||
====Acute Lead Encephalopathy==== | |||
*4 mg/kg body weight is given alone in the first dose | |||
*Thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site. | |||
*For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose. | |||
*Treatment is maintained for two to seven days depending on clinical response. *Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dimercaprol in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dimercaprol in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Dimercaprol in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Dimercaprol in adult patients. | ||
|fdaLIADPed=====Mild Arsenic or Gold Poisoning==== | |||
*2.5 mg/kg of body weight four times daily for two days. | |||
*Two times on the third day. | |||
*Only once daily thereafter for ten days. | |||
====Severe Arsenic or Gold Poisoning==== | |||
*3 mg/kg every four hours for two-days | |||
*Four times on the third day | |||
*Twice daily thereafter for ten days. | |||
====Mercury poisoning==== | |||
*5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days. | |||
====Acute Lead Encephalopathy==== | |||
*4 mg/kg body weight is given alone in the first dose | |||
*Thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site. | |||
*For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose. | |||
*Treatment is maintained for two to seven days depending on clinical response. *Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dimercaprol in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Dimercaprol in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Dimercaprol in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Dimercaprol in pediatric patients. | ||
|contraindications= | |contraindications=*Dimercaprol (Dimercaprol Injection USP) is contraindicated in most instances of [[hepatic insufficiency]] with the exception of postarsenical [[jaundice]]. | ||
|warnings=There may be local pain at the site of the injection. A reaction apparently peculiar to children is fever which may persist during therapy. It occurs in approximately 30% of children. A transient reduction of the percentage of polymorphonuclear leukocytes may also be observed. | *The drug should be discontinued or used only with extreme caution if [[acute renal insufficiency]] develops during therapy. | ||
|clinicalTrials= | |warnings=*There may be local pain at the site of the injection. | ||
*A reaction apparently peculiar to children is [[fever]] which may persist during therapy. | |||
*It occurs in approximately 30% of children. | |||
*A transient reduction of the percentage of [[polymorphonuclear leukocytes]] may also be observed. | |||
|clinicalTrials=====Cardiovascular==== | |||
*Rise in [[blood pressure]] accompanied by [[tachycardia]]. | |||
*This rise is roughly proportional to the dose administered. | |||
Doses larger than those recommended may cause other transitory signs and symptoms in approximate order of frequency as follows: | |||
====Gastrointestinal==== | |||
*[[Nausea]] | |||
*abdominal pain | |||
*in some instance, [[vomiting]] | |||
====Nervous System==== | |||
*[[Headache]] | |||
*Tingling of the hands | |||
*Burning sensation in the penis | |||
====Ophtalmology==== | |||
*[[Conjunctivitis]] | |||
*[[Lacrimation]] | |||
*Blepharal spasm | |||
====Other==== | |||
*[[Rhinorrhea]] | |||
*[[Salivation]] | |||
*[[Sweating]] of the forehead, hands and other area | |||
*Occasional appearance of painful sterile abscesses. | |||
*Burning sensation in the lips, mouth and throat | |||
*A feeling of constriction, even pain, in the throat, chest, or hands | |||
Many of the above symptoms are accompanied by a feeling of [[anxiety]], [[weakness]], and unrest and often are relieved by administration of [[antihistamine]]. | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=Animal reproduction studies have not been conducted with | |useInPregnancyFDA=Animal reproduction studies have not been conducted with dimercaprol. It is also not known whether dimercaprol can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. dimercaprol should be given to a pregnant woman only if clearly needed. | ||
It is not known whether this drug is excreted in human milk. However, because many drugs are excreted in human milk, caution should be exercised when | It is not known whether this drug is excreted in human milk. However, because many drugs are excreted in human milk, caution should be exercised when dimercaprol is administered to a nursing woman. | ||
|administration=*Intramuscular | |administration=*Intramuscular | ||
|overdose=*Dosage exceeding 5 mg/kg will usually be followed by [[vomiting]], [[convulsions]] and [[stupor]], beginning within 30 minutes and subsiding within 6 hours following injection. | |overdose=*Dosage exceeding 5 mg/kg will usually be followed by [[vomiting]], [[convulsions]] and [[stupor]], beginning within 30 minutes and subsiding within 6 hours following injection. | ||
| Line 108: | Line 176: | ||
|packLabel=[[File:Dimercaprol FDA label.png|none|450px]] | |packLabel=[[File:Dimercaprol FDA label.png|none|450px]] | ||
|alcohol=Alcohol-Dimercaprol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Dimercaprol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=*Bal In Oil | |brandNames=*Bal In Oil<ref>{{cite web|url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bee9a137-160f-47e8-a551-ca461ae4f51e|title=BAL- dimercaprol injection}} </ref> | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
| Line 116: | Line 184: | ||
|fileName=Dimercaprol package 2.png | |fileName=Dimercaprol package 2.png | ||
}} | }} | ||
Latest revision as of 04:04, 26 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dimercaprol is a heavy metal chelator that is FDA approved for the treatment of arsenic, gold and mercury poisoning. It is indicated in acute lead poisoning when used concomitantly with Edetate Calcium Disodium Injection USP. Common adverse reactions include Blepharospasm, conjunctivitis, lacrimation, nasal discharge, tightness sensation in chest, limbs, jaw and abdomen, injection site pain, nausea, vomiting, headache, paresthesia, tremor.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Mild Arsenic or Gold Poisoning
- 2.5 mg/kg of body weight four times daily for two days.
- Two times on the third day.
- Only once daily thereafter for ten days.
Severe Arsenic or Gold Poisoning
- 3 mg/kg every four hours for two-days
- Four times on the third day
- Twice daily thereafter for ten days.
Mercury poisoning
- 5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days.
Acute Lead Encephalopathy
- 4 mg/kg body weight is given alone in the first dose
- Thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site.
- For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose.
- Treatment is maintained for two to seven days depending on clinical response. *Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dimercaprol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dimercaprol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Mild Arsenic or Gold Poisoning
- 2.5 mg/kg of body weight four times daily for two days.
- Two times on the third day.
- Only once daily thereafter for ten days.
Severe Arsenic or Gold Poisoning
- 3 mg/kg every four hours for two-days
- Four times on the third day
- Twice daily thereafter for ten days.
Mercury poisoning
- 5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days.
Acute Lead Encephalopathy
- 4 mg/kg body weight is given alone in the first dose
- Thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site.
- For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose.
- Treatment is maintained for two to seven days depending on clinical response. *Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dimercaprol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dimercaprol in pediatric patients.
Contraindications
- Dimercaprol (Dimercaprol Injection USP) is contraindicated in most instances of hepatic insufficiency with the exception of postarsenical jaundice.
- The drug should be discontinued or used only with extreme caution if acute renal insufficiency develops during therapy.
Warnings
- There may be local pain at the site of the injection.
- A reaction apparently peculiar to children is fever which may persist during therapy.
- It occurs in approximately 30% of children.
- A transient reduction of the percentage of polymorphonuclear leukocytes may also be observed.
Adverse Reactions
Clinical Trials Experience
Cardiovascular
- Rise in blood pressure accompanied by tachycardia.
- This rise is roughly proportional to the dose administered.
Doses larger than those recommended may cause other transitory signs and symptoms in approximate order of frequency as follows:
Gastrointestinal
Nervous System
- Headache
- Tingling of the hands
- Burning sensation in the penis
Ophtalmology
- Conjunctivitis
- Lacrimation
- Blepharal spasm
Other
- Rhinorrhea
- Salivation
- Sweating of the forehead, hands and other area
- Occasional appearance of painful sterile abscesses.
- Burning sensation in the lips, mouth and throat
- A feeling of constriction, even pain, in the throat, chest, or hands
Many of the above symptoms are accompanied by a feeling of anxiety, weakness, and unrest and often are relieved by administration of antihistamine.
Postmarketing Experience
There is limited information regarding Dimercaprol Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Dimercaprol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Animal reproduction studies have not been conducted with dimercaprol. It is also not known whether dimercaprol can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. dimercaprol should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. However, because many drugs are excreted in human milk, caution should be exercised when dimercaprol is administered to a nursing woman.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dimercaprol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dimercaprol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Dimercaprol in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Dimercaprol in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Dimercaprol in geriatric settings.
Gender
There is no FDA guidance on the use of Dimercaprol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dimercaprol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dimercaprol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dimercaprol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dimercaprol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dimercaprol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular
Monitoring
There is limited information regarding Dimercaprol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Dimercaprol and IV administrations.
Overdosage
- Dosage exceeding 5 mg/kg will usually be followed by vomiting, convulsions and stupor, beginning within 30 minutes and subsiding within 6 hours following injection.
Pharmacology
Template:Chembox BeilsteinTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox RTECSTemplate:Chembox UNNumberTemplate:Chembox DensityTemplate:Chembox BoilingPtTemplate:Chembox LogPTemplate:Chembox pKaTemplate:Chembox pKbTemplate:Chembox RefractIndexTemplate:Chembox GHSPictogramsTemplate:Chembox GHSSignalWordTemplate:Chembox HPhrasesTemplate:Chembox PPhrasesTemplate:Chembox NFPATemplate:Chembox FlashPt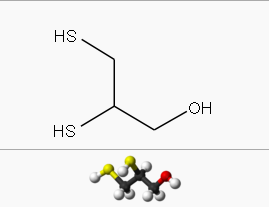
| |
| Names | |
|---|---|
| IUPAC name
2,3-Disulfanylpropan-1-ol
| |
| Other names
2,3-Dimercaptopropanol
British anti-Lewisite | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
| MeSH | Dimercaprol |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 3H 8S 2O | |
| Molar mass | 124.225 g mol-1 |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of Action
- The sulfhydryl groups of dimercaprol form complexes with certain heavy metals thus preventing or reversing the metallic binding of sulfhydryl-containing enzymes.
- The complex is excreted.
- The sustained presence of dimercaprol promotes continued excretion of the metallic poisons - arsenic, gold and mercury.
- It is also used in combination with Edetate Calcium Disodium Injection USP to promote the excretion of lead.
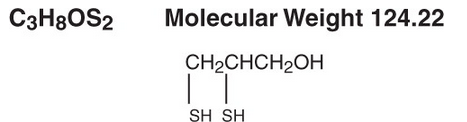
Structure
Pharmacodynamics
There is limited information regarding Dimercaprol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Dimercaprol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Dimercaprol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Dimercaprol Clinical Studies in the drug label.
How Supplied
- 3 mL (100 mg/mL) ampules, box of 10 (NDC 17478-526-03).
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Dimercaprol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dimercaprol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dimercaprol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Dimercaprol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Bal In Oil[1]
Look-Alike Drug Names
There is limited information regarding Dimercaprol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Dimercaprol |Label Name=Dimercaprol Package.png
}}
{{#subobject:
|Label Page=Dimercaprol |Label Name=Dimercaprol package 2.png
}}
- Pages with script errors
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Chemical articles with multiple PubChem CIDs
- Articles without InChI source
- Chemical articles with unknown parameter in Chembox
- ECHA InfoCard ID from Wikidata
- Chembox having DSD data
- Chembox having GHS data
- Articles containing unverified chemical infoboxes