Dibucaine: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Dibucaine": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (5 intermediate revisions by one other user not shown) | |||
| Line 4: | Line 4: | ||
|genericName=Dibucaine | |genericName=Dibucaine | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Local analgesic ointment | |drugClass=[[Local analgesia|Local analgesic ointment]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=pain and itching due to hemorrhoids or other anorectal disorders, | |indication=[[pain]] and [[itching]] due to [[hemorrhoids]] or other [[Anus|anorectal disorders]], [[Sunburn]], [[Burn|minor burns]], [[Wound|minor cuts]] | ||
|adverseReactions=unusual warmth or flushing of skin | |adverseReactions=[[Rash|unusual warmth or flushing of skin]], [[Contact dermatitis]], [[Photosensitivity]] | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
| Line 19: | Line 14: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=====Indications==== | |fdaLIADAdult=====Indications==== | ||
temporarily relieves pain and itching due to: | * temporarily relieves pain and itching due to: | ||
:* [[hemorrhoids]] or other [[Anus|anorectal disorders]], [[sunburn]], [[Burn|minor burns]], [[Wound|minor cuts]] | |||
:* scrapes, [[Insect Bites]], [[Irritation|minor skin irritation]] | |||
====Dosage==== | ====Dosage==== | ||
Directions | =====Directions===== | ||
* if possible clean the affected area with mild soap and warm water and rinse thoroughly | |||
* gently dry by patting or blotting with toilet tissue or a soft cloth before applying | |||
Adults and children 12 and over - apply externally to the affected area up to 3 to 4 times a day. | * Adults and children 12 and over - apply externally to the affected area up to 3 to 4 times a day. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 39: | Line 34: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=Children under 2 - 12 years of age - ask a doctor | |fdaLIADPed=* Children under 2 - 12 years of age - ask a doctor | ||
Infants under 2 years of age - DO NOT USE | * Infants under 2 years of age - DO NOT USE | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
| Line 51: | Line 46: | ||
|warnings=* For external use only. | |warnings=* For external use only. | ||
Allergy alert - certain persons san develop allergic reactions to ingredients in this product | * Allergy alert - certain persons san develop allergic reactions to ingredients in this product | ||
Do not use | * Do not use | ||
:* in or near the eyes | |||
:* do not get into the eyes | |||
:* in infants under 2 years of age | |||
:* in large quantities, particularly over raw surfaces or blistered areas | |||
:* do not put this product into rectum by using fingers or any mechanical device | |||
Stop use and ask a doctor if | * Stop use and ask a doctor if | ||
:* condition worsens, or does not improve within 7 days | |||
:* the symptom being treated does not subside or if [[Erythema|redness]], [[irritation]], [[swelling]], [[bleeding]] or other symptoms develop or increase | |||
If pregnant or breast feeding, as a health care professional before use | * If pregnant or breast feeding, as a health care professional before use | ||
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away | * Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 72: | Line 67: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=There is limited information regarding <i>clinical trial experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>clinical trial experience</i> of {{PAGENAME}} in the drug label. | ||
|postmarketing=unusual warmth or flushing of skin | |postmarketing=* [[Rash|unusual warmth or flushing of skin]] | ||
Chest pain | * [[Contact dermatitis]] | ||
fast or irregular heartbeat | * [[Photosensitivity]] | ||
tightness in the chest | * [[Chest pain]] | ||
|drugInteractions= | * [[Tachycardia|fast or irregular heartbeat]] | ||
* [[Chest pain|tightness in the chest]] | |||
<!--Use in Specific Populations--> | * [[Hypersensitivity reaction]] | ||
|drugInteractions=<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* If pregnant or breast feeding, as a health care professional before use | |useInPregnancyFDA=* If pregnant or breast feeding, as a health care professional before use | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 95: | Line 91: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* topical | |administration=* [[topical]] | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 104: | Line 100: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away | |overdose=* Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away | ||
|drugBox=<!--Mechanism of Action--> | |drugBox={{Drugbox2 | ||
|mechAction= | | Watchedfields = changed | ||
| verifiedrevid = 460037677 | |||
| IUPAC_name = 2-butoxy-''N''-[2-(diethylamino)ethyl]quinoline-4-carboxamide | |||
| image = Dibucaine wiki str.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|cinchocaine}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = OTC | |||
| legal_status = | |||
| routes_of_administration = topical, intravenous (equine euthanasia) | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 85-79-0 | |||
| ATC_prefix = C05 | |||
| ATC_suffix = AD04 | |||
| ATC_supplemental = {{ATC|D04|AB02}} {{ATC|N01|BB06}} {{ATC|S01|HA06}} {{ATC|S02|DA04}} | |||
| PubChem = 3025 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00527 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 2917 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = L6JW2TJG99 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00733 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 247956 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1086 | |||
<!--Chemical data--> | |||
| C=20 | H=29 | N=3 | O=2 | |||
| molecular_weight = 343.463 g/mol | |||
| smiles = O=C(c1c2ccccc2nc(OCCCC)c1)NCCN(CC)CC | |||
| InChI = 1/C20H29N3O2/c1-4-7-14-25-19-15-17(16-10-8-9-11-18(16)22-19)20(24)21-12-13-23(5-2)6-3/h8-11,15H,4-7,12-14H2,1-3H3,(H,21,24) | |||
| InChIKey = PUFQVTATUTYEAL-UHFFFAOYAU | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C20H29N3O2/c1-4-7-14-25-19-15-17(16-10-8-9-11-18(16)22-19)20(24)21-12-13-23(5-2)6-3/h8-11,15H,4-7,12-14H2,1-3H3,(H,21,24) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = PUFQVTATUTYEAL-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* Active ingredients - Dibucaine 1% | |structure=* Active ingredients - Dibucaine 1% | ||
Inactive Ingredients | * Inactive Ingredients - acetone sodium bisulfite, lanolin, light mineral oil, purified water, white petrolatum | ||
acetone sodium bisulfite, lanolin, light mineral oil, purified water, white petrolatum | |||
| Line 128: | Line 182: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
|storage=store at controlled room temperature 20 degrees to 25 degrees c | |storage=store at controlled room temperature 20 degrees to 25 degrees c | ||
|packLabel=====Ingredients and Appearance==== | |packLabel=====Ingredients and Appearance==== | ||
| Line 138: | Line 192: | ||
|fdaPatientInfo=to secure child resistant cap; screw cap tightly. Then turn cap in opposite direction. | |fdaPatientInfo=to secure child resistant cap; screw cap tightly. Then turn cap in opposite direction. | ||
If clicking sound is not heard, repeat procedure. | If clicking sound is not heard, repeat procedure. | ||
- see crimp of tube for lot number and expiration date | - see crimp of tube for lot number and expiration date | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
| Line 148: | Line 202: | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Category--> | |||
{{Vasoprotectives}} | |||
{{Antipruritics}} | |||
{{local anesthetics}} | |||
{{dermatologic-drug-stub}} | |||
{{nervous-system-drug-stub}} | |||
[[Category:Local anesthetics]] | |||
[[Category:Quinolines]] | |||
[[Category:Phenol ethers]] | |||
[[Category:Amides]] | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 19:51, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Dibucaine is a Local analgesic ointment that is FDA approved for the treatment of pain and itching due to hemorrhoids or other anorectal disorders, Sunburn, minor burns, minor cuts. Common adverse reactions include unusual warmth or flushing of skin, Contact dermatitis, Photosensitivity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- temporarily relieves pain and itching due to:
- hemorrhoids or other anorectal disorders, sunburn, minor burns, minor cuts
- scrapes, Insect Bites, minor skin irritation
Dosage
Directions
- if possible clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- Adults and children 12 and over - apply externally to the affected area up to 3 to 4 times a day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dibucaine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dibucaine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Children under 2 - 12 years of age - ask a doctor
- Infants under 2 years of age - DO NOT USE
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dibucaine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dibucaine in pediatric patients.
Contraindications
There is limited information regarding Dibucaine Contraindications in the drug label.
Warnings
- For external use only.
- Allergy alert - certain persons san develop allergic reactions to ingredients in this product
- Do not use
- in or near the eyes
- do not get into the eyes
- in infants under 2 years of age
- in large quantities, particularly over raw surfaces or blistered areas
- do not put this product into rectum by using fingers or any mechanical device
- Stop use and ask a doctor if
- condition worsens, or does not improve within 7 days
- the symptom being treated does not subside or if redness, irritation, swelling, bleeding or other symptoms develop or increase
- If pregnant or breast feeding, as a health care professional before use
- Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Adverse Reactions
Clinical Trials Experience
There is limited information regarding clinical trial experience of Dibucaine in the drug label.
Postmarketing Experience
- unusual warmth or flushing of skin
- Contact dermatitis
- Photosensitivity
- Chest pain
- fast or irregular heartbeat
- tightness in the chest
- Hypersensitivity reaction
Drug Interactions
There is limited information regarding Dibucaine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- If pregnant or breast feeding, as a health care professional before use
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dibucaine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dibucaine during labor and delivery.
Nursing Mothers
- If pregnant or breast feeding, as a health care professional before use
Pediatric Use
There is no FDA guidance on the use of Dibucaine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Dibucaine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Dibucaine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dibucaine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dibucaine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dibucaine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dibucaine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dibucaine in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Dibucaine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dibucaine in the drug label.
Overdosage
- Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Pharmacology
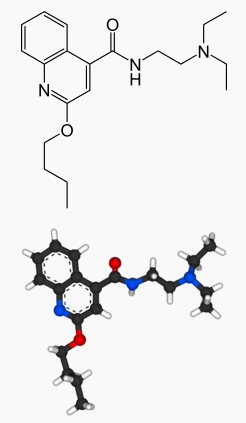
| |
Dibucaine
| |
| Systematic (IUPAC) name | |
| 2-butoxy-N-[2-(diethylamino)ethyl]quinoline-4-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | C05 D04AB02 (WHO) N01BB06 (WHO) S01HA06 (WHO) S02DA04 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 343.463 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | topical, intravenous (equine euthanasia) |
Mechanism of Action
There is limited information regarding Dibucaine Mechanism of Action in the drug label.
Structure
- Active ingredients - Dibucaine 1%
- Inactive Ingredients - acetone sodium bisulfite, lanolin, light mineral oil, purified water, white petrolatum
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dibucaine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Dibucaine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Dibucaine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Dibucaine in the drug label.
How Supplied
There is limited information regarding Dibucaine How Supplied in the drug label.
Storage
store at controlled room temperature 20 degrees to 25 degrees c
Images
Drug Images
{{#ask: Page Name::Dibucaine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Dibucaine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
to secure child resistant cap; screw cap tightly. Then turn cap in opposite direction. If clicking sound is not heard, repeat procedure. - see crimp of tube for lot number and expiration date
Precautions with Alcohol
- Alcohol-Dibucaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DIBUCAINE®[1]
Look-Alike Drug Names
There is limited information regarding the look alike names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Template:Vasoprotectives
Template:Antipruritics
Template:Dermatologic-drug-stub Template:Nervous-system-drug-stub