Cromolyn (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cromolyn (oral) is a mast cell stabilizer that is FDA approved for the treatment of mastocytosis. Common adverse reactions include headache, diarrhea, pruritus, nausea, myalgia, abdominal pain, rash, and malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL is indicated in the management of patients with mastocytosis. Use of this product has been associated with improvement in diarrhea, flushing, headaches, vomiting, urticaria, abdominal pain, nausea, and itching in some patients.
Dosage
- Adults and Adolescents (13 Years and Older): Two ampules four times daily, taken one-half hour before meals and at bedtime.
Maintenance Dose
- Once a therapeutic response has been achieved, the dose may be reduced to the minimum required to maintain the patient with a lower degree of symptomatology. To prevent relapses, the dosage should be maintained.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Cromolyn (oral) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Cromolyn (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL is indicated in the management of patients with mastocytosis. Use of this product has been associated with improvement in diarrhea, flushing, headaches, vomiting, urticaria, abdominal pain, nausea, and itching in some patients.
Dosage
- Children 2 to 12 Years: One ampule four times daily, taken one-half hour before meals and at bedtime.
- Pediatric Patients Under 2 Years: Not recommended.
- If satisfactory control of symptoms is not achieved within two to three weeks, the dosage may be increased but should not exceed 40 mg/kg/day.
- Patients should be advised that the effect of Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL therapy is dependent upon its administration at regular intervals, as directed.
Maintenance Dose
- Once a therapeutic response has been achieved, the dose may be reduced to the minimum required to maintain the patient with a lower degree of symptomatology. To prevent relapses, the dosage should be maintained.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Cromolyn (oral) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Cromolyn (oral) in pediatric patients.
Contraindications
- Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium.
Warnings
- The recommended dosage should be decreased in patients with decreased renal or hepatic function. Severe anaphylactic reactions may occur rarely in association with cromolyn sodium administration.
Adverse Reactions
Clinical Trials Experience
- Most of the adverse events reported in mastocytosis patients have been transient and could represent symptoms of the disease. The most frequently reported adverse events in mastocytosis patients who have received Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL during clinical studies were headache and diarrhea, each of which occurred in 4 of the 87 patients. Pruritus, nausea, and myalgia were each reported in 3 patients and abdominal pain, rash, and irritability in 2 patients each. One report of malaise was also recorded.
- Other Adverse Events: Additional adverse events have been reported during studies in other clinical conditions and from worldwide postmarketing experience. In most cases the available information is incomplete and attribution to the drug cannot be determined. The majority of these reports involve the gastrointestinal system and include: diarrhea, nausea, abdominal pain, constipation, dyspepsia, flatulence, glossitis, stomatitis, vomiting, dysphagia, esophagospasm.
- Other less commonly reported events (the majority representing only a single report) include the following:
- Skin: pruritus, rash, urticaria/angioedema, erythema/burning, photosensitivity.
- Musculoskeletal: arthralgia, myalgia, stiffness/weakness of legs
- Neurologic: headache, dizziness, hypoesthesia, paresthesia, migraine, convulsions, flushing
- Psychiatric: psychosis, anxiety, depression, hallucinations, behavior change, insomnia, nervousness
- Heart Rate: tachycardia, premature ventricular contractions (PVCs), palpitations
- Respiratory: pharyngitis, dyspnea
- Miscellaneous: fatigue, edema, unpleasant taste, chest pain, postprandial lightheadedness and lethargy, dysuria, urinary frequency, purpura, hepatic function test abnormal, polycythemia, neutropenia, pancytopenia, tinnitus, lupus erythematosus (LE) syndrome.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Cromolyn (oral) in the drug label.
Drug Interactions
There is limited information regarding Cromolyn (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- In reproductive studies in pregnant mice, rats, and rabbits, cromolyn sodium produced no evidence of fetal malformations at subcutaneous doses up to 540 mg/kg in mice (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis) and 164 mg/kg in rats (less than the maximum recommended daily oral dose in adults on a mg/m2 basis) or at intravenous doses up to 485 mg/kg in rabbits (approximately 4 times the maximum recommended daily oral dose in adults on a mg/m2 basis). There are, however, no adequate and well controlled studies in pregnant women.
- Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Drug Interaction During Pregnancy:
- In pregnant mice, cromolyn sodium alone did not cause significant increases in resorptions and major malformations at subcutaneous doses up to 540 mg/kg (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis). Isoproterenol alone increased both resorptions and major malformations (primarily cleft palate) at a subcutaneous dose of 2.7 mg/kg (approximately 7 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). The incidence of major malformations increased further when in cromolyn sodium at a subcutaneous dose of 540 mg/kg was added to isoproterenol at a subcutaneous dose of 2.7 mg/kg. No such interaction was observed in rats or rabbits.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cromolyn (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cromolyn (oral) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL is administered to a nursing woman.
Pediatric Use
- In adult rats no adverse effects of cromolyn sodium were observed at oral doses up to 6144 mg/kg (approximately 25 times the maximum recommended daily oral dose in adults on a mg/m2 basis). In neonatal rats, cromolyn sodium increased mortality at oral doses of 1000 mg/kg or greater (approximately 9 times the maximum recommended daily oral dose in infants on a mg/m2 basis) but not at doses of 300 mg/kg or less (approximately 3 times the maximum recommended daily oral dose in infants on a mg/m2 basis). Plasma and kidney concentrations of cromolyn after oral administration to neonatal rats were up to 20 times greater than those in older rats. In term infants up to six months of age, available clinical data suggest that the dose should not exceed 20 mg/kg/day. The use of this product in pediatric patients less than two years of age should be reserved for patients with severe disease in which the potential benefits clearly outweigh the risks.
Geriatic Use
- Clinical stuies of Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
- There is no FDA guidance on the use of Cromolyn (oral) with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Cromolyn (oral) with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Cromolyn (oral) in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Cromolyn (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Cromolyn (oral) in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Cromolyn (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL should be administered as a solution at least ½ hour before meals and at bedtime after preparation according to the following directions:
- Break open ampule(s) and squeeze liquid contents of ampule(s) into a glass of water.
- Stir solution.
- Drink all of the liquid.
Monitoring
- There is limited information regarding Monitoring of Cromolyn (oral) in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Cromolyn (oral) in the drug label.
Overdosage
There is limited information regarding Cromolyn (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the release of mediators from sensitized mast cells. Cromolyn sodium acts by inhibiting the release of histamine and leukotrienes (SRS-A) from the mast cell.
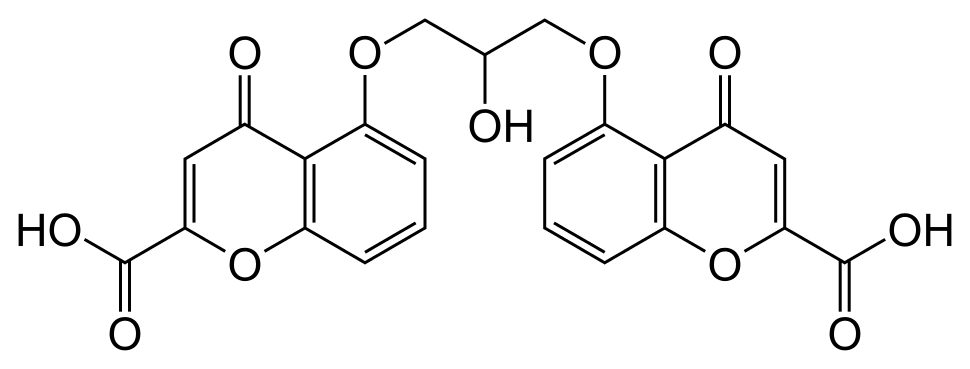
Structure
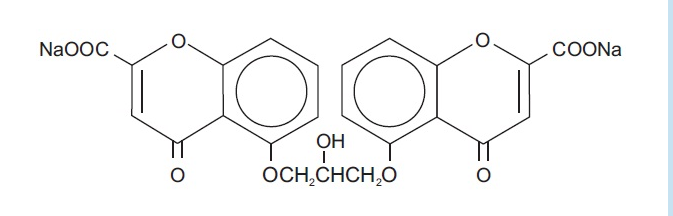
- Each 5 mL ampule of Cromolyn Sodium Oral Solution (Concentrate) contains 100 mg cromolyn sodium, USP, in Water for Injection. Cromolyn sodium is a hygroscopic, white powder having little odor. It may leave a slightly bitter aftertaste. Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL is clear colorless, and sterile. It is intended for oral use.
- Chemically, cromolyn sodium is disodium 5,5’-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]. The molecular formula is C23H14Na2O11; the molecular weight is 512.34. Its chemical structure is:
- Pharmacologic Category: Mast cell stabilizer
- Therapeutic Category: Antiallergic
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Cromolyn (oral) in the drug label.
Pharmacokinetics
- There is limited information regarding Pharmacokinetics of Cromolyn (oral) in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
- In carcinogenicity studies in mice, hamsters, and rats, cromolyn sodium had no neoplastic effects at intraperitoneal doses up to 150 mg/kg three days per week for 12 months in mice, at intraperitoneal doses up to 53 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks in hamsters, and at subcutaneous doses up to 75 mg/kg six days per week for 18 months in rats. These doses in mice, hamsters, and rats are less than the maximum recommended daily oral dose in adults and children on a mg/m2 basis.
- Cromolyn sodium showed no mutagenic potential in Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomyces cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
- In rats, cromolyn sodium showed no evidence of impaired fertility at subcutaneous doses up to 175 mg/kg in males (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis) and 100 mg/kg in females (less than the maximum recommended daily oral dose in adults on a mg/m2 basis).
Clinical Studies
- Four randomized, controlled clinical trials were conducted with Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL in patients with either cutaneous or systemic mastocytosis; two of which utilized a placebo-controlled crossover design, one utilized an active-controlled (chlorpheniramine plus cimetidine) crossover design, and one utilized a placebo-controlled parallel group design. Due to the rare nature of this disease, only 36 patients qualified for study entry, of whom 32 were considered evaluable. Consequently, formal statistical analyses were not performed. Clinically significant improvement in gastrointestinal symptoms (diarrhea, abdominal pain) were seen in the majority of patients with some improvement also seen for cutaneous manifestations (urticaria, pruritus, flushing) and cognitive function. The benefit seen with Cromolyn sodium 200 mg QID was similar to chlorpheniramine (4 mg QID) plus cimetidine (300 mg QID) for both cutaneous and systemic symptoms of mastocytosis.
- Clinical improvement occurred within 2 to 6 weeks of treatment initiation and persisted for 2 to 3 weeks after treatment withdrawal. Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL did not affect urinary histamine levels or peripheral eosinophilia, although neither of these variables appeared to correlate with disease severity. Positive clinical benefits were also reported for 37 of 51 patients who received Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL in United States and foreign humanitarian programs.
How Supplied
- Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5mL is supplied in a BFS (Blow Fill Seal) container as follows: 5 mL fill in 5 mL BFS container, with 8 containers in one rondo tray and one rondo tray in one inner carton and such 12 inner cartons in one outer carton.
- 5 mL fill in 5 mL BFS Container
- NDC 42571-132-21
- Inner Carton of 8 x 5 mL BFS Container
- NDC 42571-132-51
- Outer Carton of 96 x 5 mL (12 inner cartons of 8 x 5 mL BFS Container) NDC 42571-132-52
Storage
- Cromolyn Sodium Oral Solution (Concentrate) 100 mg/5 mL should be stored between 15°-30°C (59° to 86°F) and protected from light. Do not use if it contains a precipitate or becomes discolored. Keep out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Cromolyn (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Cromolyn (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Cromolyn (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CROMOLYN SODIUM ®[1]
Look-Alike Drug Names
There is limited information regarding Cromolyn (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Cromolyn (oral)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}