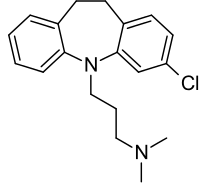
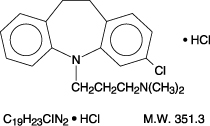
Clomipramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Suicidality and Antidepressant Drug
See full prescribing information for complete Boxed Warning.
Condition Name:
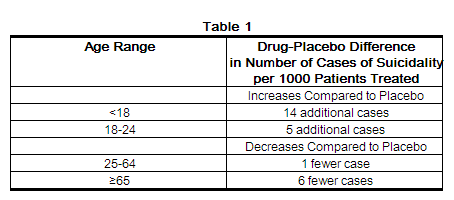
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride capsules are not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
|
Overview
Clomipramine is a Tricyclic antidepressant that is FDA approved for the {{{indicationType}}} of obsessive-compulsive disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diaphoresis, weight increased, constipation, diarrhea, indigestion, loss of appetite, nausea, xerostomia, myalgia, dizziness, feeling nervous, headache, insomnia, myoclonus, somnolence, tremor, abnormal vision, disorder of the urinary system, disorder of ejaculation, impotence, pharyngitis fatigue..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Obsessive-compulsive disorder
- Monotherapy
- Maximum, 250 mg/day[1]
- Combination Therapy
- Initial dose, 25 mg/day; weekly titration up to 75 mg/day; mean dose, 55 mg/day) plus fluoxetine (maximum dose, 40 mg/day), quetiapine initial dose, 50 mg/day; weekly titration up to 200 mg/day; mean dose, 142 mg/day
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organization)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Delusional disorder
- Initial, 25 mg/day ORALLY, may increase dosage to 100 mg/day during the first 2 weeks (MAX dose 250 mg/day, mean dose 140 mg/day)
Depression
- Initial, 75 mg/day ORALLY (3 divided doses); may increase dosage slowly as needed and tolerated to a range of 100-250 mg/day (3 divided doses)
Disorder of ejaculation
Obsessive-compulsive disorder, Intravenous therapy
- Initial, 25 mg/day ORALLY, may increase dosage to 100 mg/day ORALLY during the first 2 weeks; MAX dose 250 mg/day
Pain, chronic
Panic disorder
- 25-75 mg/day ORALLY
Pervasive developmental disorder
- There is limited information about Off-Label Non–Guideline-Supported Use of Clomipramine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Obsessive-compulsive disorder
- 10 years and older, initial, 25 mg/day ORALLY, may increase dosage to 3 mg/kg or 100 mg/day (whichever is less) ORALLY during the first 2 weeks; MAX dose 200 mg/day OR 3 mg/kg/day (whichever is less)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organization)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Depression
- Safety and effectiveness in children less than 10 years of age have not been established
- 20-30 mg/day ORALLY; may increase dosage by 10 mg/day at 4-5 day intervals as needed and tolerated
- There is limited information about Off-Label Non–Guideline-Supported Use of Clomipramine in pediatric patients.
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
|
Suicidality and Antidepressant Drug
See full prescribing information for complete Boxed Warning.
Condition Name:
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of clomipramine hydrochloride capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Clomipramine hydrochloride capsules are not approved for use in pediatric patients except for patients with obsessive compulsive disorder (OCD) (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
|
Conidition 1
Adverse Reactions
Clinical Trials Experience
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Drug Interactions
- (Drug 1)
- (Description)
- (Drug 2)
- (Description)
- (Drug 3)
- (Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Clomipramine in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clomipramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clomipramine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Clomipramine in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Clomipramine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Clomipramine in geriatric settings.
Gender
There is no FDA guidance on the use of Clomipramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clomipramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clomipramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clomipramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clomipramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clomipramine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Clomipramine Administration in the drug label.
Monitoring
There is limited information regarding Clomipramine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Clomipramine and IV administrations.
Overdosage
There is limited information regarding Clomipramine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Clomipramine Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Clomipramine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Clomipramine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Clomipramine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Clomipramine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Clomipramine How Supplied in the drug label.
Storage
There is limited information regarding Clomipramine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Clomipramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clomipramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clomipramine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Clomipramine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Clomipramine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Clomipramine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Flament MF, Rapoport JL, Berg CJ, Sceery W, Kilts C, Mellström B et al. (1985) Clomipramine treatment of childhood obsessive-compulsive disorder. A double-blind controlled study. Arch Gen Psychiatry 42 (10):977-83. PMID: 3899048
- ↑ Template:Cite isbn
{{#subobject:
|Label Page=Clomipramine |Label Name=clomipramine2.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=clomipramine3.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=Clomipramine.png.jpg
}}
{{#subobject:
|Label Page=Clomipramine |Label Name=CLOMIPRAMINE33.PNG
}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}
|fileName=}}