CitraNatal DHA: Difference between revisions
No edit summary |
m (Protected "CitraNatal DHA": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 8: | Line 8: | ||
|genericName= | |genericName= | ||
CitraNatal DHA | |||
|aOrAn= | |aOrAn= | ||
| Line 16: | Line 16: | ||
|drugClass= | |drugClass= | ||
multi-vitamin/mineral | |||
|indication= | |indication= | ||
improving the nutritional status of women prior to [[conception]], throughout [[pregnancy]], and in the postnatal period for both lactating and nonlactating mothers. | |||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
| Line 28: | Line 28: | ||
|adverseReactions= | |adverseReactions= | ||
allergic reactions | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
| Line 50: | Line 50: | ||
* Dosing Information | * Dosing Information | ||
:*CitraNatal® DHA is a multi-vitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers. | :*CitraNatal® DHA is a multi-[[vitamin]]/[[mineral]] prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers. | ||
:*One tablet and one soft gel daily or as directed by a physician. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 57: | Line 58: | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 77: | Line 64: | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 114: | Line 93: | ||
|contraindications= | |contraindications= | ||
*This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. | *This product is contraindicated in patients with a known [[hypersensitivity]] to any of the ingredients. | ||
<!--Warnings--> | <!--Warnings--> | ||
| Line 120: | Line 99: | ||
|warnings= | |warnings= | ||
*Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis. | *Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential [[antithrombotic]] effects, including an increased [[bleeding time]] and [[INR]]. Administration of omega-3 fatty acids should be avoided in patients on [[anticoagulants]] and in those known to have an inherited or acquired [[bleeding diathesis]]. | ||
*Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. | *[[Folic acid]] alone is improper therapy in the treatment of [[pernicious anemia]] and other [[megaloblastic anemias]] where [[vitamin B12]] is deficient. | ||
====Precautions==== | ====Precautions==== | ||
*Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. | *Folic acid in doses above 0.1 mg daily may obscure [[pernicious anemia]] in that hematologic remission can occur while [[neurological]] manifestations progress. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 141: | Line 120: | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 222: | Line 200: | ||
|drugBox= | |drugBox= | ||
| Line 229: | Line 206: | ||
|mechAction= | |mechAction= | ||
<!--Structure--> | <!--Structure--> | ||
| Line 238: | Line 214: | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 293: | Line 271: | ||
|brandNames= | |brandNames= | ||
* | * CitraNatal DHA®<ref>{{Cite web | title = CITRANATAL DHA- vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium | url = dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5027852-b374-10e4-7acd-42f9ada83f81 }}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
| Line 326: | Line 302: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | ||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | |||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Latest revision as of 19:11, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Accidental Overdose
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
CitraNatal DHA is a multi-vitamin/mineral that is FDA approved for the {{{indicationType}}} of improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include allergic reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Improving Nutritional status of Women
- Dosing Information
- CitraNatal® DHA is a multi-vitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.
- One tablet and one soft gel daily or as directed by a physician.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of CitraNatal DHA in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of CitraNatal DHA in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of CitraNatal DHA in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of CitraNatal DHA in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of CitraNatal DHA in pediatric patients.
Contraindications
- This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Warnings
|
Accidental Overdose
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
- Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
Precautions
- Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Adverse Reactions
Clinical Trials Experience
- Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of CitraNatal DHA in the drug label.
Drug Interactions
There is limited information regarding CitraNatal DHA Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of CitraNatal DHA in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of CitraNatal DHA during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of CitraNatal DHA with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of CitraNatal DHA with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of CitraNatal DHA with respect to geriatric patients.
Gender
There is no FDA guidance on the use of CitraNatal DHA with respect to specific gender populations.
Race
There is no FDA guidance on the use of CitraNatal DHA with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of CitraNatal DHA in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of CitraNatal DHA in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of CitraNatal DHA in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of CitraNatal DHA in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of CitraNatal DHA in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of CitraNatal DHA in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
Management
Chronic Overdose
There is limited information regarding Chronic Overdose of CitraNatal DHA in the drug label.
Pharmacology
There is limited information regarding CitraNatal DHA Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding CitraNatal DHA Mechanism of Action in the drug label.
Structure
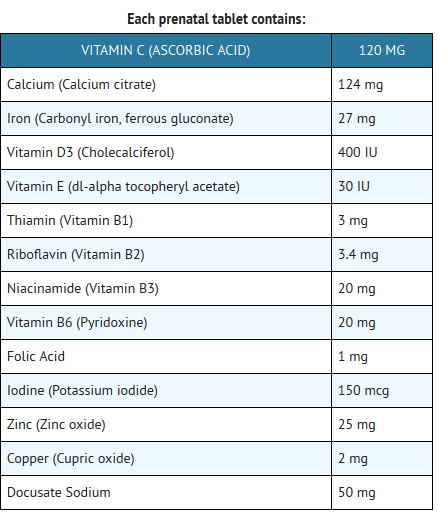
- CitraNatal® DHA is a prescription prenatal/postnatal multi-vitamin/mineral tablet with Ferr-Ease®, a patented dual-iron delivery comprising both a quick release and slow release iron, and a soft gel of an essential fatty acid. The prenatal vitamin is a white, scored, oval multi-vitamin/mineral tablet. The tablet is debossed “CN 1” on one side and is blank on the other. The essential fatty acid DHA soft gel is caramel colored and contains a light yellow to orange semi-solid mixture
Pharmacodynamics
There is limited information regarding Pharmacodynamics of CitraNatal DHA in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of CitraNatal DHA in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of CitraNatal DHA in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of CitraNatal DHA in the drug label.
How Supplied
- Six child-resistant blister packs of 5 tablets and 5 soft gels each - NDC 0178-0894-30.
- To report a serious adverse event or obtain product information, call (210) 696-8400.
- Please consult your health care provider with any dietary concerns.
- Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimalized.
- NOTICE: Contact with moisture can discolor or erode the tablet.
Storage
There is limited information regarding CitraNatal DHA Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::CitraNatal DHA |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::CitraNatal DHA |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of CitraNatal DHA in the drug label.
Precautions with Alcohol
- Alcohol-CitraNatal DHA interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CitraNatal DHA®[1]
Look-Alike Drug Names
There is limited information regarding CitraNatal DHA Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=CitraNatal DHA |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=CitraNatal DHA |Label Name=CitraNatal DHA03.png
}}
{{#subobject:
|Label Page=CitraNatal DHA |Label Name=CitraNatal DHA04.png
}}
{{#subobject:
|Label Page=CitraNatal DHA |Label Name=CitraNatal DHA05.png
}}