Chagas disease pathophysiology: Difference between revisions
Becca Cohen (talk | contribs) No edit summary |
YazanDaaboul (talk | contribs) |
||
| Line 7: | Line 7: | ||
==Pathophysiology== | ==Pathophysiology== | ||
===Transmission=== | |||
*Chagas disease usually has a vector-borne transmission. Triatomine insects, the Riduvid (kissing/assassin) bugs, suck blood from an infected individual and are subsequently infected themselves. | |||
*The insects carry the pathogen in their feces and urine. Human infection with ''T. cruzi'' occurs following exposure to feces/urine of infected insects. The pathogen typically enters the host either through a wound induced by the host's scratching following the insect bite or through the conjunctival mucus membranes. | |||
*Other modes of transmission include organ transplantation, blood transfusions, vertical transmission, breast milk, and oral transmission following ingestion of infected foods.<ref>Santos Ferreira C, Amato Neto V, Gakiya E, ''et al.'' "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321</ref><ref name=WHO>WHO. [http://www.who.int/tdr/diseases/chagas/ Chagas.] Accessed 24 September 2006.</ref><ref>da Silva Valente S, de Costa Valente V, Neto H. "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon." Mem Inst Oswaldo Cruz 94 Suppl 1: 395-8. PMID 10677763</ref><ref>UK Health Protection Agency (HPA).[http://www.hpa.org.uk/cdr/archives/archive05/News/news1305.htm Chagas’ disease (American trypanosomiasis) in southern Brazil.] Accessed 24 September 2006.</ref> | |||
The | *The incubation period following transmission is typically 1-2 weeks. | ||
[[Image:Trypanosoma cruzi LifeCycle.gif|Life cycle of [[Trypanosima cruzi]]. Source: CDC]] | [[Image:Trypanosoma cruzi LifeCycle.gif|Life cycle of [[Trypanosima cruzi]]. Source: CDC]] | ||
=== | ===Cellular Pathogenesis=== | ||
====Acute infection==== | |||
* Following transmission, trypomastigotes invade host cells via a unique transmigration process that involves bradykinin and CCL2 chemokine. | |||
*Unlike African [[trypanosomes]], the bloodstream trypomastigotes are unable to replicate. Instead, they differentiate into intracellular [[amastigote]]s intracellularly. | |||
The | * The amastigotes multiply by [[binary fission]] and re-transform into trypomastigotes, which are released again into the blood and lymphatic circulations. These newly formed trypomastigotes are then able to infect new host tissues (macrophages, fibroblasts, skeletal and cardiac myocytes, and endothelial cells), which often accounts for the high grade parasitemia and the multisystem complications associated with Chagas disease. | ||
* Inflammation is the hallmark of Chagas disease. Early host immune responses include the activation of B-cell and T-cell (CD4+ and CD8+) lymphocytes that result in the production of anti-trypanosoma antibodies and direct cytotoxicity. It is thought that host immune mechanisms simultaneously contribute to the elimination of the parasite and host tissue damage. The early response causes a state of systemic inflammation reaction that either subsides and resolves or persists as a low-grade silent inflammation and manifests as a chronic disease.<ref name="pmid24312535">{{cite journal| author=Coates BM, Sullivan DP, Makanji MY, Du NY, Olson CL, Muller WA et al.| title=Endothelial transmigration by Trypanosoma cruzi. | journal=PLoS One | year= 2013 | volume= 8 | issue= 12 | pages= e81187 | pmid=24312535 | doi=10.1371/journal.pone.0081187 | pmc=PMC3846899 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24312535 }} </ref><ref name="pmid1549177">{{cite journal| author=Tarleton RL, Koller BH, Latour A, Postan M| title=Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. | journal=Nature | year= 1992 | volume= 356 | issue= 6367 | pages= 338-40 | pmid=1549177 | doi=10.1038/356338a0 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1549177 }} </ref> | |||
====Chronic infection==== | |||
*The true mechanism that result in chronic manifestations of the disease are poorly understood, but it is thought that chronic Chagas disease may be caused by either pathogenic inflammatory responses against residual and persistent ''T. cruzi'' pathogens or autoimmune mechanisms.<ref name="pmid15275290">{{cite journal| author=Kalil J, Cunha-Neto E| title=Autoimmunity in chagas disease cardiomyopathy: Fulfilling the criteria at last? | journal=Parasitol Today | year= 1996 | volume= 12 | issue= 10 | pages= 396-9 | pmid=15275290 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15275290 }} </ref><ref name="pmid11334941">{{cite journal| author=Tarleton RL| title=Parasite persistence in the aetiology of Chagas disease. | journal=Int J Parasitol | year= 2001 | volume= 31 | issue= 5-6 | pages= 550-4 | pmid=11334941 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11334941 }} </ref><ref name="pmid10194457">{{cite journal| author=Kierszenbaum F| title=Chagas' disease and the autoimmunity hypothesis. | journal=Clin Microbiol Rev | year= 1999 | volume= 12 | issue= 2 | pages= 210-23 | pmid=10194457 | doi= | pmc=PMC88915 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10194457 }} </ref> | |||
* | *Host immune cells, namely mononuclear cells, play an important role in the chronic host tissue destruction. Other involved immune cells include activated neutrophils and eosinophils. | ||
* [[ | *Chronic inflammation typically results in end-organ damage by inducing local and diffuse tissue necrosis and fibrosis. | ||
*[[Denervation]] is characteristic of Chagas disease, whereby host organs lose [[sympathetic nervous system|sympathethic]] and [[parasympathetic nervous system|parasympathetic]] nerve endings (neuronolysis) within the walls of infected tissue. The denervation process results in clinical manifestations (e.g. GI [[hypomotility]]).<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref> | |||
===Gross Pathology=== | |||
*On goss pathology, infected organs, such as the heart, the colon, lymph nodes, or the esophagus, demonstrate the following characteristic features<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref>: | |||
:*Expansion in size | |||
:*Dilation with flabby-appearing tissue | |||
:*Tissue congestion and engorgement | |||
:*Tissue effacement and [[aneurysm]] formation (e.g. left ventricle apical effacement in Chagas cardiomyopathy) typically followed by life-threatening thrombus formation | |||
:*Whitish "soldier's" patches (early) and diffuse fibrosis (late) suggestive of tissue fibrosis in chronic states | |||
Chagas disease | ===Microscopic Pathology=== | ||
Microscopic histopathological analysis of organs infected with Chagas disease often demonstrate the following characteristic features<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref>: | |||
====Acute Phase==== | |||
*[[Pseudocyst]] formation in muscle fibers and histiocytes | |||
*[[Mononuclear]] and [[lymphocyte|lymphocytic]] cellular infiltrate | |||
*Host cell lysis (eg. [myocyte]]s, [[neuron]]s, [[endothelial cell]]s, brain [[astrocytes]]) with or without any evidence of parasite presence (especially in acute phase) | |||
*Evidence of ''T. cruzi'' parasite in the [[cytoplasm]] of host cells, such as cardiac myocytes or neurons. The parasite typically appear to be adhering to cellular [[microfilament]]s. | |||
*Palisading mononuclear cells around meningeal blood vessels (in Virchow-Robin spaces) | |||
====Indeterminate Phase==== | |||
*Low-grade inflammation in host tissue | |||
====Chronic Phase==== | |||
*Evidence of mononuclear and lymphocytic cellular infiltrate and host cell lysis | |||
*Replacement of destroyed tissue by [[fibrous scar]]s, which typically appear locally then become more diffuse | |||
==Gallery== | ==Gallery== | ||
Revision as of 15:50, 5 August 2015
|
Chagas disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chagas disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chagas disease pathophysiology |
|
Risk calculators and risk factors for Chagas disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2]
Overview
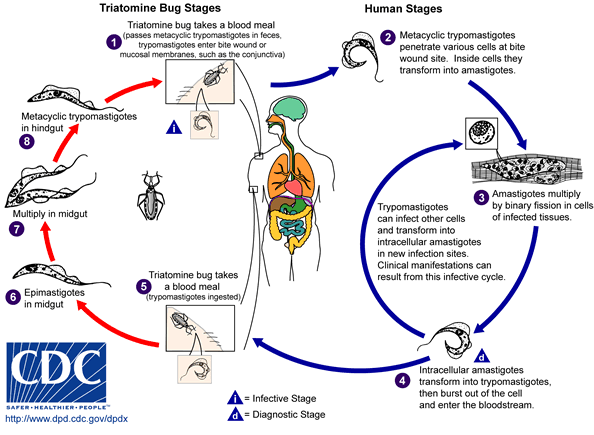
Chagas disease is caused by the protozoan Trypanosoma cruzi. T. cruzi is commonly transmitted to humans and other mammals by the blood-sucking "kissing bugs" of the subfamily Triatominae (family Reduviidae) most commonly species belonging to the Triatoma, Rhodnius, and Panstrongylus genera. It can also be transmitted through blood transfusions, organ transplantation, transplacentally, breast milk,[1] and in laboratory accidents.
Pathophysiology
Transmission
- Chagas disease usually has a vector-borne transmission. Triatomine insects, the Riduvid (kissing/assassin) bugs, suck blood from an infected individual and are subsequently infected themselves.
- The insects carry the pathogen in their feces and urine. Human infection with T. cruzi occurs following exposure to feces/urine of infected insects. The pathogen typically enters the host either through a wound induced by the host's scratching following the insect bite or through the conjunctival mucus membranes.
- Other modes of transmission include organ transplantation, blood transfusions, vertical transmission, breast milk, and oral transmission following ingestion of infected foods.[2][3][4][5]
- The incubation period following transmission is typically 1-2 weeks.
Cellular Pathogenesis
Acute infection
- Following transmission, trypomastigotes invade host cells via a unique transmigration process that involves bradykinin and CCL2 chemokine.
- Unlike African trypanosomes, the bloodstream trypomastigotes are unable to replicate. Instead, they differentiate into intracellular amastigotes intracellularly.
- The amastigotes multiply by binary fission and re-transform into trypomastigotes, which are released again into the blood and lymphatic circulations. These newly formed trypomastigotes are then able to infect new host tissues (macrophages, fibroblasts, skeletal and cardiac myocytes, and endothelial cells), which often accounts for the high grade parasitemia and the multisystem complications associated with Chagas disease.
- Inflammation is the hallmark of Chagas disease. Early host immune responses include the activation of B-cell and T-cell (CD4+ and CD8+) lymphocytes that result in the production of anti-trypanosoma antibodies and direct cytotoxicity. It is thought that host immune mechanisms simultaneously contribute to the elimination of the parasite and host tissue damage. The early response causes a state of systemic inflammation reaction that either subsides and resolves or persists as a low-grade silent inflammation and manifests as a chronic disease.[6][7]
Chronic infection
- The true mechanism that result in chronic manifestations of the disease are poorly understood, but it is thought that chronic Chagas disease may be caused by either pathogenic inflammatory responses against residual and persistent T. cruzi pathogens or autoimmune mechanisms.[8][9][10]
- Host immune cells, namely mononuclear cells, play an important role in the chronic host tissue destruction. Other involved immune cells include activated neutrophils and eosinophils.
- Chronic inflammation typically results in end-organ damage by inducing local and diffuse tissue necrosis and fibrosis.
- Denervation is characteristic of Chagas disease, whereby host organs lose sympathethic and parasympathetic nerve endings (neuronolysis) within the walls of infected tissue. The denervation process results in clinical manifestations (e.g. GI hypomotility).[11]
Gross Pathology
- On goss pathology, infected organs, such as the heart, the colon, lymph nodes, or the esophagus, demonstrate the following characteristic features[11]:
- Expansion in size
- Dilation with flabby-appearing tissue
- Tissue congestion and engorgement
- Tissue effacement and aneurysm formation (e.g. left ventricle apical effacement in Chagas cardiomyopathy) typically followed by life-threatening thrombus formation
- Whitish "soldier's" patches (early) and diffuse fibrosis (late) suggestive of tissue fibrosis in chronic states
Microscopic Pathology
Microscopic histopathological analysis of organs infected with Chagas disease often demonstrate the following characteristic features[11]:
Acute Phase
- Pseudocyst formation in muscle fibers and histiocytes
- Mononuclear and lymphocytic cellular infiltrate
- Host cell lysis (eg. [myocyte]]s, neurons, endothelial cells, brain astrocytes) with or without any evidence of parasite presence (especially in acute phase)
- Evidence of T. cruzi parasite in the cytoplasm of host cells, such as cardiac myocytes or neurons. The parasite typically appear to be adhering to cellular microfilaments.
- Palisading mononuclear cells around meningeal blood vessels (in Virchow-Robin spaces)
Indeterminate Phase
- Low-grade inflammation in host tissue
Chronic Phase
- Evidence of mononuclear and lymphocytic cellular infiltrate and host cell lysis
- Replacement of destroyed tissue by fibrous scars, which typically appear locally then become more diffuse
Gallery
-
Life cycle of Trypanosoma cruzi, the causal agent of American Trypanosomiasis. From Public Health Image Library (PHIL). [12]
References
- ↑ Santos Ferreira C, Amato Neto V, Gakiya E, et al. "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321
- ↑ Santos Ferreira C, Amato Neto V, Gakiya E, et al. "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321
- ↑ WHO. Chagas. Accessed 24 September 2006.
- ↑ da Silva Valente S, de Costa Valente V, Neto H. "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon." Mem Inst Oswaldo Cruz 94 Suppl 1: 395-8. PMID 10677763
- ↑ UK Health Protection Agency (HPA).Chagas’ disease (American trypanosomiasis) in southern Brazil. Accessed 24 September 2006.
- ↑ Coates BM, Sullivan DP, Makanji MY, Du NY, Olson CL, Muller WA; et al. (2013). "Endothelial transmigration by Trypanosoma cruzi". PLoS One. 8 (12): e81187. doi:10.1371/journal.pone.0081187. PMC 3846899. PMID 24312535.
- ↑ Tarleton RL, Koller BH, Latour A, Postan M (1992). "Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection". Nature. 356 (6367): 338–40. doi:10.1038/356338a0. PMID 1549177.
- ↑ Kalil J, Cunha-Neto E (1996). "Autoimmunity in chagas disease cardiomyopathy: Fulfilling the criteria at last?". Parasitol Today. 12 (10): 396–9. PMID 15275290.
- ↑ Tarleton RL (2001). "Parasite persistence in the aetiology of Chagas disease". Int J Parasitol. 31 (5–6): 550–4. PMID 11334941.
- ↑ Kierszenbaum F (1999). "Chagas' disease and the autoimmunity hypothesis". Clin Microbiol Rev. 12 (2): 210–23. PMC 88915. PMID 10194457.
- ↑ 11.0 11.1 11.2 Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA (2006). "Chagas disease". Postgrad Med J. 82 (974): 788–98. doi:10.1136/pgmj.2006.047357. PMC 2653922. PMID 17148699.
- ↑ "Public Health Image Library (PHIL)".
![Life cycle of Trypanosoma cruzi, the causal agent of American Trypanosomiasis. From Public Health Image Library (PHIL). [12]](/images/9/9e/Chagas03.jpeg)