Cerliponase alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cerliponase alfa is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Brineura is contraindicated in patients with:
- acute intraventricular access device-related complications (e.g., leakage, device failure, or device-related infection).
- ventriculoperitoneal shunts.
Warnings
Intraventricular Access Device‑Related Complications
- Brineura must be administered using aseptic technique to reduce the risk of infection. Healthcare professionals should inspect the scalp for skin integrity to ensure the intraventricular access device is not compromised prior to each infusion.
- Brineura is contraindicated if there are signs of acute intraventricular access device-related complications (e.g., leakage, device failure or signs of device-related infection such as swelling, erythema of the scalp, extravasation of fluid, or bulging of the scalp around or above the intraventricular access device). In case of intraventricular access device complications, discontinue the Brineura infusion and refer to the device manufacturer’s labeling for further instructions.
- The signs and symptoms of device-related infections may not be apparent, therefore, CSF samples should routinely be sent for testing to detect subclinical device infections.
- In clinical studies with Brineura, intraventricular access device-related infections were observed in two patients. In each case, antibiotics were administered, the intraventricular access device was replaced, and the patient continued on Brineura treatment.
- Material degradation of the intraventricular access device reservoir may occur after approximately 105 perforations of the intraventricular access device. The intraventricular access device may require replacement as soon as, or prior to, 105 administrations of Brineura, equating to approximately 4.3 years of regular administrations.
Cardiovascular Adverse Reactions
- Monitor vital signs (blood pressure, heart rate) before infusion starts, periodically during infusion, and post-infusion in a healthcare setting. Upon completion of the infusion, clinically assess the patient status. Continued observation may be necessary if clinically indicated.
- Perform electrocardiogram (ECG) monitoring during infusion in patients with a history of bradycardia, conduction disorder, or with structural heart disease, as some patients with CLN2 disease may develop conduction disorders or heart disease. In patients without cardiac abnormalities, regular 12-lead ECG evaluations should be performed every 6 months.
- In the clinical studies, hypotension was reported in 2 (8%) patients, which occurred during or up to eight hours after Brineura infusion. Patients did not require alteration in treatment, and reactions resolved spontaneously or after intravenous fluid administration.
Hypersensitivity Reactions
- Hypersensitivity reactions have been reported in Brineura-treated patients during the clinical studies. A total of 11 (46%) patients experienced hypersensitivity reactions during the infusion or within 24 hours of completion of the infusion. The signs and symptoms observed concomitantly with hypersensitivity reactions included pyrexia, vomiting, pleocytosis or irritability. Patients were routinely pre-medicated with antihistamines with or without antipyretics or corticosteroids, prior to infusion of Brineura.
- Due to the potential for anaphylaxis, appropriate medical support should be readily available when Brineura is administered. If anaphylaxis occurs, immediately discontinue the infusion and initiate appropriate medical treatment. Observe patients closely during and after the infusion. Inform patients/caregivers of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs and symptoms occur.
- The management of hypersensitivity reactions should be based on the severity of the reaction and may include temporarily interrupting the infusion, and/or treatment with antihistamines, antipyretics, and/or corticosteroids. If a severe hypersensitivity reaction occurs, immediately discontinue the infusion and initiate appropriate medical treatment.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
There is limited information regarding Cerliponase alfa Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no available data on Brineura use in pregnant women to inform a drug-associated risk of pregnancy-related outcomes. Animal reproduction studies have not been conducted using cerliponase alfa.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cerliponase alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cerliponase alfa during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of cerliponase alfa in human milk, the effects on the breastfed child, or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Brineura to an infant during lactation; therefore, the development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Brineura and any potential adverse effects on the breastfed infant from Brineura or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness of Brineura have been established in pediatric patients 3 years of age and older. Pediatric use of Brineura to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency, is supported by a non-randomized single-arm dose escalation clinical study with extension in patients with CLN2 disease and compared to untreated patients with CLN2 disease from an independent natural history cohort [see Clinical Studies (14)]. Safety and effectiveness in patients less than 3 years of age have not been established.
Geriatic Use
There is no FDA guidance on the use of Cerliponase alfa in geriatric settings.
Gender
There is no FDA guidance on the use of Cerliponase alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cerliponase alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cerliponase alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cerliponase alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cerliponase alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cerliponase alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
- Brineura and the Intraventricular Electrolytes must only be administered by the intraventricular route, using the provided Administration Kit for use with Brineura. Each vial of Brineura and Intraventricular Electrolytes is intended for a single dose only.
- Each infusion consists of 10 mL of Brineura followed by 2 mL of Intraventricular Electrolytes. The complete infusion must be administered using an infusion set with a 0.2 micron inline filter. The Intraventricular Electrolytes are used to flush the infusion line, port needle, and intraventricular access device in order to fully administer Brineura and to maintain patency of the intraventricular access device.
Monitoring
- Decreased loss of ambulation may indicate clinical efficacy.
- CSF testing: Routinely to identify device-related infection.
- Scalp skin integrity: Prior to infusion.
- Signs of acute intraventricular access device-related complications (eg, device leakage, failure, or infection): Prior to infusion.
- Blood pressure and heart rate: Prior to infusion, periodically during infusion, and after infusion; continue observation if clinically indicated.
- ECG: During infusion in patients with structural heart disease, conduction disorder, or history of bradycardia; every 6 months in patients with normal cardiac function.
- Signs and symptoms of anaphylaxis: During and after infusion.
IV Compatibility
There is limited information regarding the compatibility of Cerliponase alfa and IV administrations.
Overdosage
There is limited information regarding Cerliponase alfa overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- CLN2 disease is a neurodegenerative disease caused by deficiency of the lysosomal enzyme tripeptidyl peptidase-1 (TPP1), which catabolizes polypeptides in the CNS. TPP1 has no known substrate specificity. Deficiency in TPP1 activity results in the accumulation of lysosomal storage materials normally metabolized by this enzyme in the central nervous system (CNS), leading to progressive decline in motor function.
- Cerliponase alfa (rhTTP1), a proenzyme, is taken up by target cells in the CNS and is translocated to the lysosomes through the Cation Independent Mannose-6-Phosphate Receptor (CI-MPR, also known as M6P/IGF2 receptor). Cerliponase alfa is activated in the lysosome and the activated proteolytic form of rhTPP1 cleaves tripeptides from the N-terminus of proteins.
Structure
There is limited information regarding Cerliponase alfa Structure in the drug label.
Pharmacodynamics
There is limited information regarding Cerliponase alfa Pharmacodynamics in the drug label.
Pharmacokinetics
- The pharmacokinetics of cerliponase alfa were evaluated in patients with CLN2 disease who received intraventricular infusions of 30 mg (0.1 times the approved recommended dosage), 100 mg (approximately 0.3 times the approved recommended dosage), and 300 mg over approximately 4.5 hours once every other week.
- Cerliponase alfa CSF exposure following the initial single dose administration of Brineura increased less than proportionally across doses of 30 mg, 100 mg, and 300 mg. There was no apparent accumulation of cerliponase alfa in CSF or plasma when Brineura was administered at a dose of 300 mg once every other week.
- Cerliponase alfa pharmacokinetics have high inter-subject and intra-subject variability. Following intraventricular infusions of 300 mg of Brineura at Day 1, Week 5, and Week 13, the pharmacokinetic parameters in CSF and plasma were assessed in 14 patients and are summarized in Table 2.

Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity, genotoxicity, and fertility studies have not been performed with cerliponase alfa. Based on the mechanism of action, cerliponase alfa is not expected to be tumorigenic.
Clinical Studies
- The efficacy of Brineura was assessed over 96 weeks in a non-randomized single-arm dose escalation clinical study with extension in symptomatic pediatric patients with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease, confirmed by TPP1 deficiency. Brineura-treated patients were compared to untreated patients from a natural history cohort. The Motor domain of a CLN2 Clinical Rating Scale was used to assess disease progression. Scores ranged from 3 (grossly normal) to 0 (profoundly impaired) with unit decrements representing milestone events in the loss of motor function (ability to walk or crawl). Due to the inability to establish comparability for the CLN2 Language domain ratings between the clinical study with extension and the natural history cohort, efficacy of Brineura for the Language domain cannot be established.
- Twenty-four patients, aged 3 to 8 years were enrolled in the Brineura single-arm clinical study. Sixty-three percent of patients were female and 37% were male. Ninety-six percent of patients were Caucasian and 4% were Asian. One patient withdrew after week 1 due to inability to continue with study procedures; 23 patients were treated with Brineura 300 mg every other week for 48 weeks, and continued treatment during the extension period.
- In the clinical study with extension, patients were assessed for decline in the Motor domain of the CLN2 Clinical Rating Scale at 48, 72 and 96 weeks. Decline was defined as having an unreversed (sustained) 2-category decline or an unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale. Patients’ responses to Brineura treatment were evaluated if at screening a combined Motor plus Language CLN2 score of less than 6 was recorded. Two patients with a combined Motor plus Language CLN2 score of 6 were excluded from the analyses; they maintained that score throughout the study period. The patient who terminated early was analyzed as having a decline at the time of termination. Data used in the analyses from the natural history cohort began at 36 months of age or greater and at the first time a Motor plus Language CLN2 score less than 6 was recorded.
- Motor scores of the 22 Brineura-treated patients in the clinical study with extension were compared to scores of the independent natural history cohort that included 42 untreated patients who satisfied inclusion criteria for the clinical study. The results of logistic modeling with covariates (screening age, screening motor score, genotype: 0 key mutations (yes/no)), demonstrated the odds of Brineura-treated patients not having a decline by 96 weeks were 13 times the odds of natural history cohort patients not having a decline (Odds Ratio (95% CI): 13.1 (1.2, 146.9)).
Descriptive non-randomized comparison
- In an unadjusted non-randomized comparison, of the 22 patients treated with Brineura and evaluated for efficacy at week 96, 21 (95%) did not decline, and only the patient who terminated early was deemed to have a decline in the Motor domain of the CLN2 Clinical Rating Scale. Results from the natural history cohort demonstrated progressive decline in motor function; of the 42 patients in the natural history cohort, 21 (50%) experienced an unreversed (sustained) 2-category decline or unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale over 96 weeks.
- Given the non-randomized study design, a Cox Proportional Hazards Model adjusted for age, initial motor score, and genotype was used to evaluate time to unreversed 2-category decline or unreversed score of 0 in the Motor domain. This model showed a lesser decrease in motor function in the Brineura-treated patients when compared to the natural history cohort (see Figure 7).

Motor Domain Scores: Matched Patients Only
- To further assess efficacy, the 22 patients from the Brineura clinical study with a baseline combined Motor plus Language CLN2 score less than 6 were matched to 42 patients in the natural history cohort. Patients were matched based on the following covariates: baseline age at time of screening within 3 months, genotype (0, 1, or 2 key mutations), and baseline Motor domain CLN2 score at time of screening.
- Using the Motor domain of the CLN2 Clinical Rating Scale, decline was defined as having an unreversed 2-category decline or an unreversed score of 0. At 96 weeks, the matched analysis based on 17 pairs demonstrated fewer declines in the Motor domain for Brineura-treated patients compared to untreated patients in the natural history cohort (see Table 3).

How Supplied
- Brineura is supplied as a sterile, clear to slightly opalescent and colorless to pale yellow solution for intraventricular infusion and Intraventricular Electrolytes Injection is supplied as a clear to colorless solution for intraventricular infusion; both are included in package 1 of 2. The Administration Kit for use with Brineura is supplied separately as package 2 of 2.
Package 1 of 2
- Each Brineura (cerliponase alfa) Injection vial has a green flip‑off cap (plastic), and contains 150 mg cerliponase alfa per 5 mL (30 mg/mL).
- Each Intraventricular Electrolytes Injection vial has a yellow flip‑off cap (plastic), and contains 5 mL of solution.

Package 2 of 2
- The Administration Kit for use with Brineura is supplied separately and contains the following single-use, sterile infusion components:
- Two 20-mL syringes.
- Two syringe needles (21 G, 25.4 mm).
- One extension line.
- One infusion set with 0.2 micron inline filter.
- One port needle (22 G, 16 mm).
Storage
- Brineura (cerliponase alfa) Injection and Intraventricular Electrolytes Injection:
- Store upright in a freezer (‑25°C to ‑15°C) in original carton to protect from light.
- Administration Kit for use with Brineura:
- Store in original carton separately from Brineura. Do not freeze.
Images
Drug Images
{{#ask: Page Name::Cerliponase alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

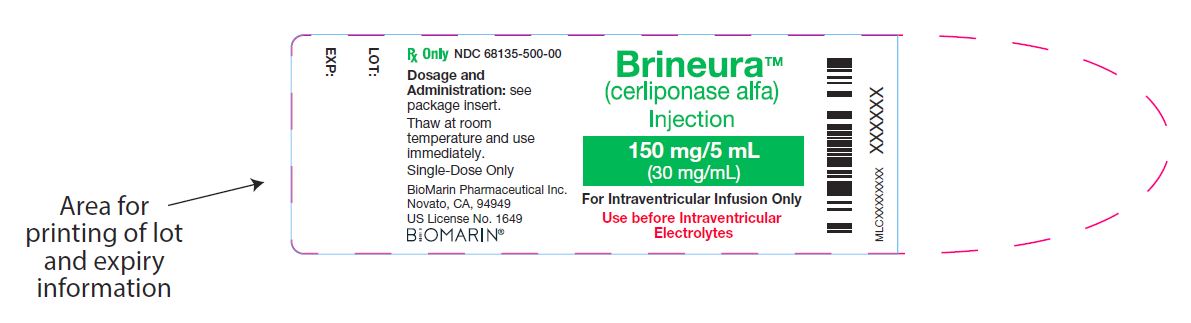


{{#ask: Label Page::Cerliponase alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Intraventricular Access Device‑Related Complications
- Advise patients and caregivers of the risk of device-related infections. If any signs of infection are present, instruct patients to immediately contact their healthcare provider.
Cardiovascular Adverse Reactions
- Advise patients and caregivers that hypotension and/or bradycardia may occur during and following the infusion of Brineura. Instruct patients immediately to contact their healthcare provider if these reactions occur.
Hypersensitivity Reactions
- Advise patients and caregivers that hypersensitivity reactions related to Brineura treatment, including fever, vomiting, and irritability may occur. Due to the potential for anaphylaxis, inform patients and caregivers of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs and symptoms occur.
Precautions with Alcohol
Alcohol-Cerliponase alfa interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Brineura
Look-Alike Drug Names
There is limited information regarding Cerliponase alfa Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.