COVID-19-associated respiratory failure: Difference between revisions
Sara Mohsin (talk | contribs) |
Sara Mohsin (talk | contribs) |
||
| (25 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{COVID-19}} | {{SI}} | ||
{{Main article|COVID-19}} | |||
'''For COVID-19 frequently asked inpatient questions, click [[COVID-19 frequently asked inpatient questions|here]]'''<br> | |||
'''For COVID-19 frequently asked outpatient questions, click [[COVID-19 frequently asked outpatient questions|here]]'''<br>'''For COVID-19 patient information, click [[COVID-19 (patient information)|here]]''' | |||
{{CMG}}; {{AE}} {{Usman Ali Akbar}} | {{CMG}}; {{AE}} {{Usman Ali Akbar}} | ||
{{SK}}2019 novel coronavirus disease, COVID19,Wuhan | {{SK}}2019 novel coronavirus disease, COVID19, Wuhan virus, Type 1 respiratory failure, Hypoxemic respiratory failure | ||
==Overview== | ==Overview== | ||
The most devastating [[Complication (medicine)|complication]] of [[SARS-CoV-2]] is [[Respiratory failure|acute hypoxaemic respiratory failure]] which requires [[mechanical ventilation]]. There can be multiple causes that can lead to acute hypoxemic [[respiratory failure]] ranging from [[pulmonary edema]] , vascular occlusion, ventilation/perfusion mismatch and hemoglobinopathies. The most important pathology is usually [[acute respiratory distress syndrome]] (ARDS) that eventually leads to hypoxemic [[respiratory failure]] in patients that are not maintaining [[oxygen saturation]]. The difference between COVID related ARDS and the usual ARDS is the injury site which is mainly the respiratory system involving the alveolar epithelial cells. Usually the clinical symptoms don't correlate with the severity of imaging and laboratory findings. [[Compliance|Lung compliance]] | The most devastating [[Complication (medicine)|complication]] of [[SARS-CoV-2]] is [[Respiratory failure|acute hypoxaemic respiratory failure]] which requires [[mechanical ventilation]]. There can be multiple causes that can lead to acute hypoxemic [[respiratory failure]] ranging from [[pulmonary edema]], vascular occlusion, ventilation/perfusion mismatch, and hemoglobinopathies. The most important pathology is usually [[acute respiratory distress syndrome]] (ARDS) that eventually leads to hypoxemic [[respiratory failure]] in patients that are not maintaining [[oxygen saturation]]. The difference between COVID related ARDS and the usual ARDS is the injury site which is mainly the respiratory system involving the alveolar epithelial cells. Usually, the clinical symptoms don't correlate with the severity of imaging and laboratory findings. [[Compliance|Lung compliance]] may be normal in some patients. The patients that progress towards [[Respiratory failure|acute respiratory failure]] are usually managed by mechanical intubation with [[tidal volume]] target of 6mL/kg PBW. | ||
[[File:Covid-resp-failure.jpg|700px|center]] | |||
==Historical Perspective== | ==Historical Perspective== | ||
* In December 2019, an outbreak of [[COVID-19|coronavirus disease]] (COVID-19) broke out in Wuhan,China. | * In December 2019, an outbreak of [[COVID-19|coronavirus disease]] (COVID-19) broke out in Wuhan, China. | ||
* COVID-19 was a clustering [[pneumonia]] like illness which affected patients that rapidly developed ARDS. | * COVID-19 was a clustering [[pneumonia]] like illness which affected patients that rapidly developed ARDS. | ||
* ARDS | * ARDS is one of the most important causes of hospital and ICU admission due to COVID. | ||
* Many [[autopsies]] studies reported [[Acute respiratory distress syndrome|ARDS]] to be the cause of death in patients dying due to respiratory complications of COVID. | * Many [[autopsies]] studies reported [[Acute respiratory distress syndrome|ARDS]] to be the cause of death in patients dying due to respiratory complications of COVID. | ||
==Classification== | ==Classification== | ||
* [[Respiratory failure]] can be classified | * [[Respiratory failure]] can be classified into two types. | ||
* Primarily, COVID-19 associated respiratory failure is [[type 1 respiratory failure]] which is actually hypoxemic respiratory failure. | * Primarily, COVID-19 associated respiratory failure is [[type 1 respiratory failure]] which is actually hypoxemic respiratory failure.<ref name="Li Ma p. ">{{cite journal | last=Li | first=Xu | last2=Ma | first2=Xiaochun | title=Acute respiratory failure in COVID-19: is it “typical” ARDS? | journal=Critical Care | publisher=Springer Science and Business Media LLC | volume=24 | issue=1 | date=2020-05-06 | issn=1364-8535 | doi=10.1186/s13054-020-02911-9 | page=}}</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
|+Respiratory Failure Types | |+Respiratory Failure Types | ||
| Line 45: | Line 50: | ||
*[[Hypothyroidism]] | *[[Hypothyroidism]] | ||
|} | |} | ||
==Pathophysiology== | ==Pathophysiology== | ||
* After the infection of the [[SARS-CoV-2]] virus, [[Pneumocytes|Type II pneumocytes]] release inflammatory [[cytokines]] that recruit [[Macrophage|macrophages.]] | * After the infection of the [[SARS-CoV-2]] virus, [[Pneumocytes|Type II pneumocytes]] release inflammatory [[cytokines]] that recruit [[Macrophage|macrophages.]]<ref name="Chen Guo Pan Zhao 2020 pp. 135–140">{{cite journal | last=Chen | first=Yun | last2=Guo | first2=Yao | last3=Pan | first3=Yihang | last4=Zhao | first4=Zhizhuang Joe | title=Structure analysis of the receptor binding of 2019-nCoV | journal=Biochemical and Biophysical Research Communications | publisher=Elsevier BV | volume=525 | issue=1 | year=2020 | issn=0006-291X | doi=10.1016/j.bbrc.2020.02.071 | pages=135–140}}</ref> | ||
*[[Macrophage|Macrophages]] further release [[cytokines]] that cause [[vasodilation]] and allow more [[neutrophils]] and [[Macrophage|macrophages]] to come to the site of injury. | *[[Macrophage|Macrophages]] further release [[cytokines]] that cause [[vasodilation]] and allow more [[neutrophils]] and [[Macrophage|macrophages]] to come to the site of injury. | ||
* Fluid starts accumulating into the alveolus which leads to dilution of [[surfactant]]. | * Fluid starts accumulating into the alveolus which leads to dilution of [[surfactant]]. | ||
| Line 55: | Line 59: | ||
* Normally, type II cells are the precursor cells for type I cells. | * Normally, type II cells are the precursor cells for type I cells. | ||
* This postulated sequence of events has been further supported by the murine model of [[influenza]] pneumonia. | * This postulated sequence of events has been further supported by the murine model of [[influenza]] pneumonia. | ||
* The pathological result of SARS and COVID-19 is [[diffuse alveolar damage]] in which there is the development of fibrin [[Hyaline membrane disease|hyaline membranes]] and formation of a few [[multinucleated giant cells]]. This aberrant wound healing may lead to further [[scarring]] and [[fibrosis]] which is more severe than other forms of ARDS. | * The pathological result of SARS and COVID-19 is [[diffuse alveolar damage]] in which there is the development of fibrin [[Hyaline membrane disease|hyaline membranes]] and formation of a few [[multinucleated giant cells]]. This aberrant wound healing may lead to further [[scarring]] and [[fibrosis]] which is more severe than other forms of ARDS.<ref name="Mason 2020 p=2000607">{{cite journal | last=Mason | first=Robert J. | title=Pathogenesis of COVID-19 from a cell biology perspective | journal=European Respiratory Journal | publisher=European Respiratory Society (ERS) | volume=55 | issue=4 | year=2020 | issn=0903-1936 | doi=10.1183/13993003.00607-2020 | page=2000607}}</ref> | ||
* Recovery will require a vigorous [[Innate immune response|innate]] and [[Acquired immunity|acquired]] immune response and epithelial regeneration. | * Recovery will require a vigorous [[Innate immune response|innate]] and [[Acquired immunity|acquired]] immune response and epithelial regeneration. | ||
* Elderly individuals are particularly susceptible because of their diminished immune response and inability to regenerate damaged [[epithelium]] quickly. Due to reduced mucociliary clearance, it may allow the virus to spread to the gas exchange units of the lung more readily. | * Elderly individuals are particularly susceptible because of their diminished immune response and inability to regenerate damaged [[epithelium]] quickly. Due to reduced mucociliary clearance, it may allow the virus to spread to the gas exchange units of the lung more readily. | ||
* The [[respiratory failure]] resulting as a complication of [[Acute respiratory distress syndrome|ARDS]] is due to the result of the acute systemic inflammatory response which is caused by the direct or indirect insults to the lungs. | * The [[respiratory failure]] resulting as a complication of [[Acute respiratory distress syndrome|ARDS]] is due to the result of the acute systemic inflammatory response which is caused by the direct or indirect insults to the lungs.<ref name="Mason 2020 p=2000607">{{cite journal | last=Mason | first=Robert J. | title=Pathogenesis of COVID-19 from a cell biology perspective | journal=European Respiratory Journal | publisher=European Respiratory Society (ERS) | volume=55 | issue=4 | year=2020 | issn=0903-1936 | doi=10.1183/13993003.00607-2020 | page=2000607}}</ref> | ||
* In the early exudative stage of [[Acute respiratory distress syndrome|ARDS]], there occurs the development of [[Acute respiratory distress syndrome|diffuse alveolar damage]] with the destruction of [[epithelial cells]] and [[Endothelial cell|endothelial cells.]] | * In the early exudative stage of [[Acute respiratory distress syndrome|ARDS]], there occurs the development of [[Acute respiratory distress syndrome|diffuse alveolar damage]] with the destruction of [[epithelial cells]] and [[Endothelial cell|endothelial cells.]] | ||
*There are two mechanisms mentioned in the table below which can lead [[Acute respiratory distress syndrome|ARDS]] or any other pathology to respiratory failure in COVID-19: | *There are two mechanisms mentioned in the table below which can lead [[Acute respiratory distress syndrome|ARDS]] or any other pathology to respiratory failure in COVID-19: | ||
| Line 73: | Line 77: | ||
| | | | ||
*[[Shunt (medical)|Shunt]] cannot be corrected despite 100 % oxygen. | *[[Shunt (medical)|Shunt]] cannot be corrected despite 100 % oxygen. | ||
* The deoxygenated blood bypasses the well-ventilated alveoli and mixes with the blood that has been passed through the ventilated alveoli . | * The deoxygenated blood bypasses the well-ventilated alveoli and mixes with the blood that has been passed through the ventilated alveoli. | ||
* This leads to a reduction in arterial blood content. Hypoxemic [[Shunt (medical)|shunts]] are difficult to correct through 100 % oxygen. | * This leads to a reduction in arterial blood content. Hypoxemic [[Shunt (medical)|shunts]] are difficult to correct through 100 % oxygen. | ||
|} | |} | ||
==Causes== | ==Causes== | ||
*Airspace filling in acute hypoxemic [[respiratory failure]] (AHRF) is due to the following: | *Airspace filling in acute hypoxemic [[respiratory failure]] (AHRF) is due to the following <ref name="Zucker 1988 pp. 813–30">{{cite journal | last=Zucker | first=AR | title=Therapeutic strategies for acute hypoxemic respiratory failure. | journal=Critical care clinics | volume=4 | issue=4 | year=1988 | issn=0749-0704 | pmid=3052710 | pages=813–30}}</ref>: | ||
**Elevated alveolar-capillary hydrostatic pressure. | **Elevated alveolar-capillary hydrostatic pressure. | ||
**Increased alveolar-capillary permeability such as [[COVID-19]] predisposing to [[acute respiratory distress syndrome]] (ARDS). | **Increased alveolar-capillary permeability such as [[COVID-19]] predisposing to [[acute respiratory distress syndrome]] (ARDS). | ||
| Line 86: | Line 90: | ||
The [[Acute respiratory distress syndrome|ARDS]] and associated subsequent [[respiratory failure]] caused by [[COVID-19]] can be differentiated by other diseases based on the following three factors:<ref name="Li Ma p.">{{cite journal | last=Li | first=Xu | last2=Ma | first2=Xiaochun | title=Acute respiratory failure in COVID-19: is it “typical” ARDS? | journal=Critical Care | publisher=Springer Science and Business Media LLC | volume=24 | issue=1 | date=2020-05-06 | issn=1364-8535 | doi=10.1186/s13054-020-02911-9 | page=}}</ref> | The [[Acute respiratory distress syndrome|ARDS]] and associated subsequent [[respiratory failure]] caused by [[COVID-19]] can be differentiated by other diseases based on the following three factors:<ref name="Li Ma p.">{{cite journal | last=Li | first=Xu | last2=Ma | first2=Xiaochun | title=Acute respiratory failure in COVID-19: is it “typical” ARDS? | journal=Critical Care | publisher=Springer Science and Business Media LLC | volume=24 | issue=1 | date=2020-05-06 | issn=1364-8535 | doi=10.1186/s13054-020-02911-9 | page=}}</ref> | ||
* '''Timing of Onset :''' According to Berlin Criteria of [[Acute respiratory distress syndrome|ARDS]] , the onset must be within 1 week of clinical insult but various reported onsets of [[COVID-19]] related ARDS had mean onset time of 8-12 days from [[Acute respiratory distress syndrome|ARDS]]. It is therefore suggested that there should be caution against development of [[Acute respiratory distress syndrome|ARDS]] in COVID-19 patients with the course of more than week.<ref name="Li Ma p." /> | * '''Timing of Onset:''' According to Berlin Criteria of [[Acute respiratory distress syndrome|ARDS]], the onset must be within 1 week of clinical insult but various reported onsets of [[COVID-19]] related ARDS had mean onset time of 8-12 days from [[Acute respiratory distress syndrome|ARDS]]. It is therefore suggested that there should be caution against the development of [[Acute respiratory distress syndrome|ARDS]] in COVID-19 patients with the course of more than a week.<ref name="Li Ma p." /> | ||
* '''Respiratory system compliance :''' Not all the cases of [[acute respiratory failure]] caused by COVID-19 were ARDS.The typical CT findings of the COVID-19 affected patient showed bilateral ground-glass shadow with a peripheral lung distribution. Although there were [[Consolidation (medicine)|consolidation]] and exudation, this was not the typical "ARDS picture".Lung [[compliance]] might be relatively normal in some COVID-19-related ARDS patients who met ARDS Berlin criteria which is inconsistent with ARDS caused by other factors.In addition, the lung compliance was relatively high in some COVID-19-related ARDS patients, which was inconsistent with the severity of [[hypoxemia]]. | * '''Respiratory system compliance:''' Not all the cases of [[acute respiratory failure]] caused by COVID-19 were ARDS. The typical CT findings of the COVID-19 affected patient showed bilateral ground-glass shadow with a peripheral lung distribution. Although there were [[Consolidation (medicine)|consolidation]] and exudation, this was not the typical "ARDS picture".Lung [[compliance]] might be relatively normal in some COVID-19-related ARDS patients who met ARDS Berlin criteria which is inconsistent with ARDS caused by other factors. In addition, the lung compliance was relatively high in some COVID-19-related ARDS patients, which was inconsistent with the severity of [[hypoxemia]]. | ||
* '''Severity of Hypoxemia''' : | * '''Severity of Hypoxemia''' : | ||
| Line 108: | Line 112: | ||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
*In a sampling of some of the larger epidemiologic studies of patients with COVID-19 to date, rates of invasive mechanical ventilation among patients admitted to ICUs leading to [[respiratory failure]] range from 29.1% in one Chinese study | *In a sampling of some of the larger epidemiologic studies of patients with COVID-19 to date, rates of invasive mechanical ventilation among patients admitted to ICUs leading to [[respiratory failure]] range from 29.1% in one Chinese study to 89.9% in a U.S. study and anywhere from 2.3% of patients admitted to the hospital up to 33.1%.<ref name="American Journal of Respiratory and Critical Care Medicine 2020 p.">{{cite journal | title=Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology | journal=American Journal of Respiratory and Critical Care Medicine | date=2020-07-01 | url=https://www.atsjournals.org/doi/10.1164/rccm.202004-1385ED# | access-date=2020-07-02 | page=}}</ref> | ||
* The epidemiological data regarding age, gender and race of patients developing ARDS and subsequent [[respiratory failure]] is insufficient. | *The epidemiological data regarding age, gender, and race of patients developing ARDS and subsequent [[respiratory failure]] is insufficient. | ||
==Risk Factors== | ==Risk Factors== | ||
*Comorbidities and other conditions that have been associated with severe illness and progression of [[Acute respiratory distress syndrome|ARDS]] to subsequent respiratory failure include:<ref name="Liu Fang Deng Liu 2020 pp. 1025–1031">{{cite journal | last=Liu | first=Kui | last2=Fang | first2=Yuan-Yuan | last3=Deng | first3=Yan | last4=Liu | first4=Wei | last5=Wang | first5=Mei-Fang | last6=Ma | first6=Jing-Ping | last7=Xiao | first7=Wei | last8=Wang | first8=Ying-Nan | last9=Zhong | first9=Min-Hua | last10=Li | first10=Cheng-Hong | last11=Li | first11=Guang-Cai | last12=Liu | first12=Hui-Guo | title=Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province | journal=Chinese medical journal | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=133 | issue=9 | date=2020-05-05 | issn=0366-6999 | pmid=32044814 | pmc=7147277 | doi=10.1097/cm9.0000000000000744 | pages=1025–1031}}</ref> <ref name="Arentz Yim Klaff Lokhandwala p=1612">{{cite journal | last=Arentz | first=Matt | last2=Yim | first2=Eric | last3=Klaff | first3=Lindy | last4=Lokhandwala | first4=Sharukh | last5=Riedo | first5=Francis X. | last6=Chong | first6=Maria | last7=Lee | first7=Melissa | title=Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State | journal=JAMA | publisher=American Medical Association (AMA) | volume=323 | issue=16 | date=2020-04-28 | issn=0098-7484 | pmid=32191259 | pmc=7082763 | doi=10.1001/jama.2020.4326 | page=1612}}</ref> <ref name="Wu Chen Cai Xia p.">{{cite journal | last=Wu | first=Chaomin | last2=Chen | first2=Xiaoyan | last3=Cai | first3=Yanping | last4=Xia | first4=Jia’an | last5=Zhou | first5=Xing | last6=Xu | first6=Sha | last7=Huang | first7=Hanping | last8=Zhang | first8=Li | last9=Zhou | first9=Xia | last10=Du | first10=Chunling | last11=Zhang | first11=Yuye | last12=Song | first12=Juan | last13=Wang | first13=Sijiao | last14=Chao | first14=Yencheng | last15=Yang | first15=Zeyong | last16=Xu | first16=Jie | last17=Zhou | first17=Xin | last18=Chen | first18=Dechang | last19=Xiong | first19=Weining | last20=Xu | first20=Lei | last21=Zhou | first21=Feng | last22=Jiang | first22=Jinjun | last23=Bai | first23=Chunxue | last24=Zheng | first24=Junhua | last25=Song | first25=Yuanlin | title=Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China | journal=JAMA internal medicine | publisher=American Medical Association (AMA) | date=2020-03-13 | issn=2168-6106 | pmid=32167524 | pmc=7070509 | doi=10.1001/jamainternmed.2020.0994 | page=}}</ref> | *Comorbidities and other conditions that have been associated with severe illness and progression of [[Acute respiratory distress syndrome|ARDS]] to subsequent respiratory failure include:<ref name="Liu Fang Deng Liu 2020 pp. 1025–1031">{{cite journal | last=Liu | first=Kui | last2=Fang | first2=Yuan-Yuan | last3=Deng | first3=Yan | last4=Liu | first4=Wei | last5=Wang | first5=Mei-Fang | last6=Ma | first6=Jing-Ping | last7=Xiao | first7=Wei | last8=Wang | first8=Ying-Nan | last9=Zhong | first9=Min-Hua | last10=Li | first10=Cheng-Hong | last11=Li | first11=Guang-Cai | last12=Liu | first12=Hui-Guo | title=Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province | journal=Chinese medical journal | publisher=Ovid Technologies (Wolters Kluwer Health) | volume=133 | issue=9 | date=2020-05-05 | issn=0366-6999 | pmid=32044814 | pmc=7147277 | doi=10.1097/cm9.0000000000000744 | pages=1025–1031}}</ref><ref name="Arentz Yim Klaff Lokhandwala p=1612">{{cite journal | last=Arentz | first=Matt | last2=Yim | first2=Eric | last3=Klaff | first3=Lindy | last4=Lokhandwala | first4=Sharukh | last5=Riedo | first5=Francis X. | last6=Chong | first6=Maria | last7=Lee | first7=Melissa | title=Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State | journal=JAMA | publisher=American Medical Association (AMA) | volume=323 | issue=16 | date=2020-04-28 | issn=0098-7484 | pmid=32191259 | pmc=7082763 | doi=10.1001/jama.2020.4326 | page=1612}}</ref><ref name="Wu Chen Cai Xia p.">{{cite journal | last=Wu | first=Chaomin | last2=Chen | first2=Xiaoyan | last3=Cai | first3=Yanping | last4=Xia | first4=Jia’an | last5=Zhou | first5=Xing | last6=Xu | first6=Sha | last7=Huang | first7=Hanping | last8=Zhang | first8=Li | last9=Zhou | first9=Xia | last10=Du | first10=Chunling | last11=Zhang | first11=Yuye | last12=Song | first12=Juan | last13=Wang | first13=Sijiao | last14=Chao | first14=Yencheng | last15=Yang | first15=Zeyong | last16=Xu | first16=Jie | last17=Zhou | first17=Xin | last18=Chen | first18=Dechang | last19=Xiong | first19=Weining | last20=Xu | first20=Lei | last21=Zhou | first21=Feng | last22=Jiang | first22=Jinjun | last23=Bai | first23=Chunxue | last24=Zheng | first24=Junhua | last25=Song | first25=Yuanlin | title=Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China | journal=JAMA internal medicine | publisher=American Medical Association (AMA) | date=2020-03-13 | issn=2168-6106 | pmid=32167524 | pmc=7070509 | doi=10.1001/jamainternmed.2020.0994 | page=}}</ref> | ||
**Age appears (major risk factor predicting progression to ARDS) | **Age appears (major risk factor predicting progression to ARDS) | ||
**High fever (≥39°C) | **High fever (≥39°C) | ||
| Line 127: | Line 131: | ||
==Screening== | ==Screening== | ||
* There is insufficient evidence to recommend routine screening for [[COVID-19|COVID]] associated [[respiratory failure]]. | * There is insufficient evidence to recommend routine screening for [[COVID-19|COVID]] associated [[respiratory failure]]. | ||
==Natural History, Complications, and Prognosis== | ==Natural History, Complications, and Prognosis== | ||
| Line 136: | Line 140: | ||
=== '''Complications''' === | === '''Complications''' === | ||
* The complications that | * The complications that result from mechanical ventilation which is often required in the patients developing respiratory failure include: | ||
**[[Barotrauma]] | **[[Barotrauma]] | ||
** Ventilation associated lung injury | ** Ventilation associated lung injury | ||
** Intrinsic [[Positive end-expiratory pressure|positive end expiratory pressure]] | ** Intrinsic [[Positive end-expiratory pressure|positive end-expiratory pressure]] | ||
** Heterogeneous [[ventilation]] | ** Heterogeneous [[ventilation]] | ||
** Altered [[Ventilation-perfusion mismatch|V/Q mismatch]] | ** Altered [[Ventilation-perfusion mismatch|V/Q mismatch]] | ||
| Line 148: | Line 152: | ||
* The mortality from COVID-19 appears driven by the presence of severe [[Acute respiratory distress syndrome|ARDS]] and subsequent [[respiratory failure]] is approximately 50 percent (range 12 to 78 percent). <ref name="Chen Zhou Dong Qu 2020 pp. 507–513">{{cite journal | last=Chen | first=Nanshan | last2=Zhou | first2=Min | last3=Dong | first3=Xuan | last4=Qu | first4=Jieming | last5=Gong | first5=Fengyun | last6=Han | first6=Yang | last7=Qiu | first7=Yang | last8=Wang | first8=Jingli | last9=Liu | first9=Ying | last10=Wei | first10=Yuan | last11=Xia | first11=Jia'an | last12=Yu | first12=Ting | last13=Zhang | first13=Xinxin | last14=Zhang | first14=Li | title=Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study | journal=The Lancet | publisher=Elsevier BV | volume=395 | issue=10223 | year=2020 | issn=0140-6736 | doi=10.1016/s0140-6736(20)30211-7 | pages=507–513}}</ref> | * The mortality from COVID-19 appears driven by the presence of severe [[Acute respiratory distress syndrome|ARDS]] and subsequent [[respiratory failure]] is approximately 50 percent (range 12 to 78 percent). <ref name="Chen Zhou Dong Qu 2020 pp. 507–513">{{cite journal | last=Chen | first=Nanshan | last2=Zhou | first2=Min | last3=Dong | first3=Xuan | last4=Qu | first4=Jieming | last5=Gong | first5=Fengyun | last6=Han | first6=Yang | last7=Qiu | first7=Yang | last8=Wang | first8=Jingli | last9=Liu | first9=Ying | last10=Wei | first10=Yuan | last11=Xia | first11=Jia'an | last12=Yu | first12=Ting | last13=Zhang | first13=Xinxin | last14=Zhang | first14=Li | title=Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study | journal=The Lancet | publisher=Elsevier BV | volume=395 | issue=10223 | year=2020 | issn=0140-6736 | doi=10.1016/s0140-6736(20)30211-7 | pages=507–513}}</ref> | ||
* Other risk factors associated with death among critically ill patients include development of severe [[Acute respiratory distress syndrome|ARDS]] and requirement of [[mechanical ventilation]], comorbidities, increased markers of inflammation, worsening [[Lymphocytopenia|lymphopenia]],[[neutrophilia]] and [[troponin]] leak. | * Other risk factors associated with death among critically ill patients include the development of severe [[Acute respiratory distress syndrome|ARDS]] and requirement of [[mechanical ventilation]], comorbidities, increased markers of inflammation, worsening [[Lymphocytopenia|lymphopenia]],[[neutrophilia]] and [[troponin]] leak. | ||
==Diagnosis== | ==Diagnosis== | ||
===Diagnostic Study of Choice=== | ===Diagnostic Study of Choice=== | ||
* There are no established criteria for the diagnosis of COVID associated [[respiratory failure]]. | * There are no established criteria for the diagnosis of COVID-19 associated [[respiratory failure]]. | ||
* Caution is observed when a patient develops [[Acute respiratory distress syndrome|ARDS]] and oxygen saturation is maintained with HFNC and non-invasive [[Ventilation (physiology)|ventilation]]. | * Caution is observed when a patient develops [[Acute respiratory distress syndrome|ARDS]] and oxygen saturation is maintained with HFNC and non-invasive [[Ventilation (physiology)|ventilation]]. | ||
* [[Respiratory failure]] is set into play when there is SpO2 sat <90% despite maximal supplemental oxygen. | * [[Respiratory failure]] is set into play when there is SpO2 sat <90% despite maximal supplemental oxygen. | ||
| Line 165: | Line 169: | ||
===Physical Examination=== | ===Physical Examination=== | ||
*[[Cyanosis]] can be seen on general physical exam. | *[[Cyanosis]] can be seen on a general physical exam. | ||
* Altered level of [[sensorium]] or consciousness. | * Altered level of [[sensorium]] or consciousness. | ||
*[[Crackles]] during chest auscultation which are worse at lung bases. | *[[Crackles]] during chest auscultation which are worse at lung bases. | ||
| Line 193: | Line 197: | ||
* When compared to the PF ratio, the A-a gradient is found to correlate less well with pulmonary shunting.<ref name="pmid6409506">{{cite journal |author=Covelli HD, Nessan VJ, Tuttle WK |title=Oxygen derived variables in acute respiratory failure |journal=Crit. Care Med. |volume=11 |issue=8 |pages=646–9 |year=1983 |pmid=6409506 |doi=}}</ref><ref name="pmid14769743">{{cite journal |author=El-Khatib MF, Jamaleddine GW |title=A new oxygenation index for reflecting intrapulmonary shunting in patients undergoing open-heart surgery |journal=Chest |volume=125 |issue=2 |pages=592–6 |year=2004 |pmid=14769743 |doi=}}</ref><ref name="pmid3191742">{{cite journal |author=Cane RD, Shapiro BA, Templin R, Walther K |title=Unreliability of oxygen tension-based indices in reflecting intrapulmonary shunting in critically ill patients |journal=Crit. Care Med. |volume=16 |issue=12 |pages=1243–5 |year=1988 |pmid=3191742 |doi=}}</ref> | * When compared to the PF ratio, the A-a gradient is found to correlate less well with pulmonary shunting.<ref name="pmid6409506">{{cite journal |author=Covelli HD, Nessan VJ, Tuttle WK |title=Oxygen derived variables in acute respiratory failure |journal=Crit. Care Med. |volume=11 |issue=8 |pages=646–9 |year=1983 |pmid=6409506 |doi=}}</ref><ref name="pmid14769743">{{cite journal |author=El-Khatib MF, Jamaleddine GW |title=A new oxygenation index for reflecting intrapulmonary shunting in patients undergoing open-heart surgery |journal=Chest |volume=125 |issue=2 |pages=592–6 |year=2004 |pmid=14769743 |doi=}}</ref><ref name="pmid3191742">{{cite journal |author=Cane RD, Shapiro BA, Templin R, Walther K |title=Unreliability of oxygen tension-based indices in reflecting intrapulmonary shunting in critically ill patients |journal=Crit. Care Med. |volume=16 |issue=12 |pages=1243–5 |year=1988 |pmid=3191742 |doi=}}</ref> | ||
* Particular lab findings associated with worse prognosis include: | * Particular lab findings associated with a worse prognosis include: | ||
**[[Lymphopenia]] | **[[Lymphopenia]] | ||
**[[Fever elevated liver enzymes|Elevated liver enzymes]] | **[[Fever elevated liver enzymes|Elevated liver enzymes]] | ||
| Line 223: | Line 227: | ||
** Perihilar infiltrates | ** Perihilar infiltrates | ||
[[File:Covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards.jpg|300px|thumb|none|Bilateral alveolar consolidation with panlobar change, with typical radiological findings of ARDS | [[File:Covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards.jpg|300px|thumb|none|Bilateral alveolar consolidation with panlobar change, with typical radiological findings of ARDS. [https://radiopaedia.org/cases/covid-19-rapidly-progressive-acute-respiratory-distress-syndrome-ards?lang=us Source: Dr. Edgar Lorente]]] | ||
===CT scan=== | ===CT scan=== | ||
| Line 229: | Line 233: | ||
* There are no CT scan findings associated with respiratory failure due to COVID-19. | * There are no CT scan findings associated with respiratory failure due to COVID-19. | ||
* However, a CT scan may be helpful in the diagnosis of complications of [[coronavirus]] which include the Ground glass (GGO) pattern which is a common finding in COVID-19 patients with following characteristics: | * However, a CT scan may be helpful in the diagnosis of complications of [[coronavirus]] which include the Ground glass (GGO) pattern which is a common finding in COVID-19 patients with following characteristics: | ||
**Multifocal (GGO presents as a unifocal lesion in the early phase of disease). | **Multifocal (GGO presents as a unifocal lesion in the early phase of the disease). | ||
**[[Bilateral]] | **[[Bilateral]] | ||
**Peripheral | **Peripheral | ||
| Line 249: | Line 253: | ||
* There is no treatment for respiratory failure due to COVID-19; the mainstay of therapy is supportive care and mechanical ventilation. | * There is no treatment for respiratory failure due to COVID-19; the mainstay of therapy is supportive care and mechanical ventilation. | ||
=== Indications of Tracheal intubation and Mechanical Ventilation | ===Indications of Tracheal intubation and Mechanical Ventilation=== | ||
* Following is the list of indications for tracheal intubation and mechanical ventilation in COVID-19 | * Following is the list of indications for tracheal intubation and mechanical ventilation in COVID-19 associated respiratory failure:<ref name="Alanazi 2015 p=99">{{cite journal | last=Alanazi | first=Abdullah | title=Intubations and airway management: An overview of Hassles through third millennium | journal=Journal of Emergencies, Trauma, and Shock | publisher=Medknow | volume=8 | issue=2 | year=2015 | issn=0974-2700 | doi=10.4103/0974-2700.145401 | page=99}}</ref> | ||
*Signs of [[respiratory distress]] | **Signs of [[respiratory distress]] such as: | ||
* Rapid progression of the disease | ***Accessory muscle use | ||
* SpO2 sat <90% despite maximal supplemental oxygen | ***Paradoxical abdominal breathing) | ||
* Arterial pH <7.3 with [[PaCO2]] >50 | **Rapid progression of the disease | ||
* Patient requiring >40 L/minute HFNC and FiO2 >0.6 | **SpO2 sat <90% despite maximal supplemental oxygen | ||
* Hemodynamic instability; [[Multiple organ dysfunction syndrome|multiorgan failure]] | **Arterial pH <7.3 with [[PaCO2]] >50 | ||
**Patient requiring >40 L/minute HFNC and FiO2 >0.6 | |||
**Hemodynamic instability; [[Multiple organ dysfunction syndrome|multiorgan failure]] | |||
=== Ventilator settings === | === Ventilator settings === | ||
*Following is the list of ventilator settings required in COVID-19 associated respiratory failure:<ref name="NHLBI ARDS Network">{{cite web | title=About | website=NHLBI ARDS Network | url=http://www.ardsnet.org/ | language=it | access-date=2020-07-02}}</ref> | |||
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
! style="background: #4479BA; width: 200px;" |{{fontcolor|#FFF|Low Tidal Volume Ventilation is recommended | ! style="background: #4479BA; width: 200px;" |{{fontcolor|#FFF|Low Tidal Volume Ventilation is recommended}} | ||
|- | |- | ||
| | | | ||
| Line 280: | Line 287: | ||
=== RECOVERY Trial === | === RECOVERY Trial === | ||
* It has been recommended in RECOVERY Trial that for patients requiring mechanical ventilation or those requiring oxygen should be given low dose [[dexamethasone]] (6mg daily for 10 days) for ICU patients. | * It has been recommended in RECOVERY Trial that for patients requiring mechanical ventilation or those requiring oxygen should be given low dose [[dexamethasone]] (6mg daily for 10 days) for ICU patients.<ref name="pmid32678530">{{cite journal| author=RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL | display-authors=etal| title=Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. | journal=N Engl J Med | year= 2020 | volume= | issue= | pages= | pmid=32678530 | doi=10.1056/NEJMoa2021436 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32678530 }} </ref> | ||
* It has been shown to reduce the mortality rate by 1/3 rd in mechanically ventilated patients.<ref name="RECOVERY Trial 2020">{{cite web | title=Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19 | website=RECOVERY Trial | date=2020-06-16 | url=https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19 | access-date=2020-07-02}}</ref> | * It has been shown to reduce the mortality rate by 1/3 rd in mechanically ventilated patients.<ref name="RECOVERY Trial 2020">{{cite web | title=Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19 | website=RECOVERY Trial | date=2020-06-16 | url=https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19 | access-date=2020-07-02}}</ref> | ||
[[File: | [[File:Corel-draw-pneumonia-mechanical vent-temp.jpg|800px|center]] | ||
==Prevention== | ==Prevention== | ||
| Line 302: | Line 309: | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Up-To-Date]] | |||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 17:56, 27 July 2020
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
For COVID-19 patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Usman Ali Akbar, M.B.B.S.[2]
Synonyms and keywords:2019 novel coronavirus disease, COVID19, Wuhan virus, Type 1 respiratory failure, Hypoxemic respiratory failure
Overview
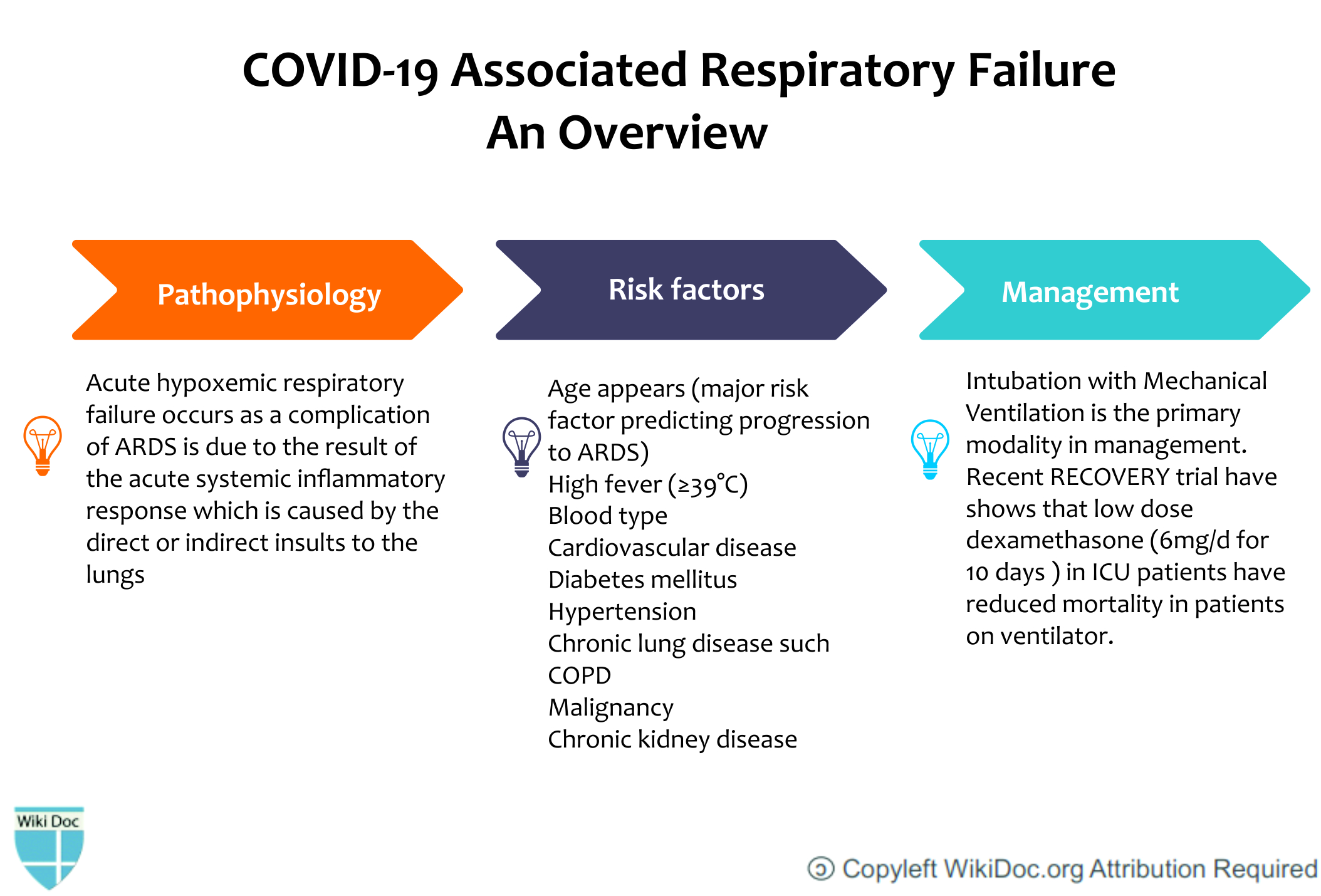
The most devastating complication of SARS-CoV-2 is acute hypoxaemic respiratory failure which requires mechanical ventilation. There can be multiple causes that can lead to acute hypoxemic respiratory failure ranging from pulmonary edema, vascular occlusion, ventilation/perfusion mismatch, and hemoglobinopathies. The most important pathology is usually acute respiratory distress syndrome (ARDS) that eventually leads to hypoxemic respiratory failure in patients that are not maintaining oxygen saturation. The difference between COVID related ARDS and the usual ARDS is the injury site which is mainly the respiratory system involving the alveolar epithelial cells. Usually, the clinical symptoms don't correlate with the severity of imaging and laboratory findings. Lung compliance may be normal in some patients. The patients that progress towards acute respiratory failure are usually managed by mechanical intubation with tidal volume target of 6mL/kg PBW.
Historical Perspective
- In December 2019, an outbreak of coronavirus disease (COVID-19) broke out in Wuhan, China.
- COVID-19 was a clustering pneumonia like illness which affected patients that rapidly developed ARDS.
- ARDS is one of the most important causes of hospital and ICU admission due to COVID.
- Many autopsies studies reported ARDS to be the cause of death in patients dying due to respiratory complications of COVID.
Classification
- Respiratory failure can be classified into two types.
- Primarily, COVID-19 associated respiratory failure is type 1 respiratory failure which is actually hypoxemic respiratory failure.[1]
| Hypoxemic Respiratory Failure (Type 1) | Hypoxemic Respiratory Failure (Type 2) |
|---|---|
| PaO2 is lower than 60 mmHg | PaCO2 is greater than 50 mmHg |
| Most common form of respiratory failure | Mostly seen in COPD patients |
|
Other Etiologies may include
|
Pathophysiology
- After the infection of the SARS-CoV-2 virus, Type II pneumocytes release inflammatory cytokines that recruit macrophages.[2]
- Macrophages further release cytokines that cause vasodilation and allow more neutrophils and macrophages to come to the site of injury.
- Fluid starts accumulating into the alveolus which leads to dilution of surfactant.
- SARS-CoV-2 propagates within type II cells and releases a large number of viral particles. The type II pneumocytes undergo apoptosis and die. This results in a self-replicating pulmonary toxin as the released viral particles infect type II cells in adjacent units.
- Normally, type II cells are the precursor cells for type I cells.
- This postulated sequence of events has been further supported by the murine model of influenza pneumonia.
- The pathological result of SARS and COVID-19 is diffuse alveolar damage in which there is the development of fibrin hyaline membranes and formation of a few multinucleated giant cells. This aberrant wound healing may lead to further scarring and fibrosis which is more severe than other forms of ARDS.[3]
- Recovery will require a vigorous innate and acquired immune response and epithelial regeneration.
- Elderly individuals are particularly susceptible because of their diminished immune response and inability to regenerate damaged epithelium quickly. Due to reduced mucociliary clearance, it may allow the virus to spread to the gas exchange units of the lung more readily.
- The respiratory failure resulting as a complication of ARDS is due to the result of the acute systemic inflammatory response which is caused by the direct or indirect insults to the lungs.[3]
- In the early exudative stage of ARDS, there occurs the development of diffuse alveolar damage with the destruction of epithelial cells and endothelial cells.
- There are two mechanisms mentioned in the table below which can lead ARDS or any other pathology to respiratory failure in COVID-19:
| V/Q Mismatch | Development of Shunt |
|---|---|
|
|
Causes
- Airspace filling in acute hypoxemic respiratory failure (AHRF) is due to the following [4]:
- Elevated alveolar-capillary hydrostatic pressure.
- Increased alveolar-capillary permeability such as COVID-19 predisposing to acute respiratory distress syndrome (ARDS).
- Hemorrhage into alveoli or inflammatory exudates.
Differentiating COVID-19-associated respiratory failure from other Diseases
The ARDS and associated subsequent respiratory failure caused by COVID-19 can be differentiated by other diseases based on the following three factors:[1]
- Timing of Onset: According to Berlin Criteria of ARDS, the onset must be within 1 week of clinical insult but various reported onsets of COVID-19 related ARDS had mean onset time of 8-12 days from ARDS. It is therefore suggested that there should be caution against the development of ARDS in COVID-19 patients with the course of more than a week.[1]
- Respiratory system compliance: Not all the cases of acute respiratory failure caused by COVID-19 were ARDS. The typical CT findings of the COVID-19 affected patient showed bilateral ground-glass shadow with a peripheral lung distribution. Although there were consolidation and exudation, this was not the typical "ARDS picture".Lung compliance might be relatively normal in some COVID-19-related ARDS patients who met ARDS Berlin criteria which is inconsistent with ARDS caused by other factors. In addition, the lung compliance was relatively high in some COVID-19-related ARDS patients, which was inconsistent with the severity of hypoxemia.
- Severity of Hypoxemia :
| Berlin Criteria Of ARDS leading to respiratory failure | COVID-Related ARDS leading to respiratory failure |
|---|---|
ARDS is divided into three stages based on oxygenation index (PaO2/FiO2) on PEEP ≥ 5 cmH2O:[5]
|
COVID-19-related ARDS was divided into three categories based on oxygenation index (PaO2/FiO2) on PEEP ≥ 5 cmH2O [6]
|
Epidemiology and Demographics
- In a sampling of some of the larger epidemiologic studies of patients with COVID-19 to date, rates of invasive mechanical ventilation among patients admitted to ICUs leading to respiratory failure range from 29.1% in one Chinese study to 89.9% in a U.S. study and anywhere from 2.3% of patients admitted to the hospital up to 33.1%.[7]
- The epidemiological data regarding age, gender, and race of patients developing ARDS and subsequent respiratory failure is insufficient.
Risk Factors
- Comorbidities and other conditions that have been associated with severe illness and progression of ARDS to subsequent respiratory failure include:[8][9][10]
- Age appears (major risk factor predicting progression to ARDS)
- High fever (≥39°C)
- Blood type
- Cardiovascular disease
- Diabetes mellitus
- Hypertension
- Chronic lung disease such COPD
- Malignancy
- Chronic kidney disease
- Obesity
- Smoking
Screening
- There is insufficient evidence to recommend routine screening for COVID associated respiratory failure.
Natural History, Complications, and Prognosis
- The development of ARDS and subsequent respiratory failure is a major transition from conservative medical therapy to mechanical ventilation in the management of COVID-19.
- Various case studies show the development of respiratory failure an eventual outcome in patients developing moderate to severe symptoms eventually requiring mechanical ventilation.
Complications
- The complications that result from mechanical ventilation which is often required in the patients developing respiratory failure include:
- Barotrauma
- Ventilation associated lung injury
- Intrinsic positive end-expiratory pressure
- Heterogeneous ventilation
- Altered V/Q mismatch
- Diaphragmatic muscle atrophy
- Respiratory muscle weakness
Prognosis
- The mortality from COVID-19 appears driven by the presence of severe ARDS and subsequent respiratory failure is approximately 50 percent (range 12 to 78 percent). [11]
- Other risk factors associated with death among critically ill patients include the development of severe ARDS and requirement of mechanical ventilation, comorbidities, increased markers of inflammation, worsening lymphopenia,neutrophilia and troponin leak.
Diagnosis
Diagnostic Study of Choice
- There are no established criteria for the diagnosis of COVID-19 associated respiratory failure.
- Caution is observed when a patient develops ARDS and oxygen saturation is maintained with HFNC and non-invasive ventilation.
- Respiratory failure is set into play when there is SpO2 sat <90% despite maximal supplemental oxygen.
- Chest X-ray and arterial blood gas measurement is done periodically to check for the development of ARDS.
History and Symptoms
- The patient usually has a history of PCR positive COVID-19 and has already developed symptoms of ARDS which include difficulty in breathing and failure to maintain oxygen saturation on room air.
- The patient may also present with restlessness, anxiety, loss of consciousness, rapid and shallow breathing, racing heart , irregular heartbeats,and profuse sweating.
Physical Examination
- Cyanosis can be seen on a general physical exam.
- Altered level of sensorium or consciousness.
- Crackles during chest auscultation which are worse at lung bases.
- Jugular venous distention with high levels of end-expiratory pressure.
Laboratory Findings
- An arterial blood gas is mandatory to confirm the diagnosis of respiratory failure.
- One needs to document two of the three criteria to formally diagnose acute respiratory failure: pO2 less than 60 mm Hg (or room air oxygen saturation less than or equal to 90%), pCO2 greater than 50 mm Hg with pH less than 7.35, and signs/symptoms of respiratory distress.
Tests of Oxygenation
- PaO2/FiO2 ratio (PF ratio)
- <math>{P/F\ ratio} = \left (\frac{PaO_2}{Fi0_2}\right) \times 100</math>
- Normal is 500
- ARDS is < 200
- This measure is easier to calculate.
- Comparative studies suggest it correlates better with pulmonary shunts than does the A-a gradient.[12][13][14]
- Alveolar-arterial oxygen (A-a) gradient (alveolar-arterial oxygen difference - AVO2D)
- <math>\mbox{A-a gradient} = {PAO_2}\ -\ {PaO_2}</math>
- <math>{PAO_2} = {Fi0_2} *\left ({760 - 47}\right) \ -\ \frac{PaCO_2}{0.8}</math>
- Normal is < 10 mm Hg
- Although, it is difficult to calculate A-a gradient and also it relies on the respiratory quotient being constant in the prediction of alveolar CO2 but it still accounts for changes in the respiration as measured by the partial pressure of carbon dioxide.
- When compared to the PF ratio, the A-a gradient is found to correlate less well with pulmonary shunting.[12][13][14]
- Particular lab findings associated with a worse prognosis include:
- Lymphopenia
- Elevated liver enzymes
- Elevated lactate dehydrogenase
- Elevated inflammatory markers
- Elevated D-dimer (>1 mcg/mL)
- Elevated prothrombin time (PT)
- Elevated troponin
- Elevated creatine phosphokinase (CPK)
Electrocardiogram
- There are no ECG findings associated with COVID associated respiratory failure.
Echocardiography or Ultrasound
- There are no echocardiography/ultrasound findings associated with COVID associated respiratory failure.
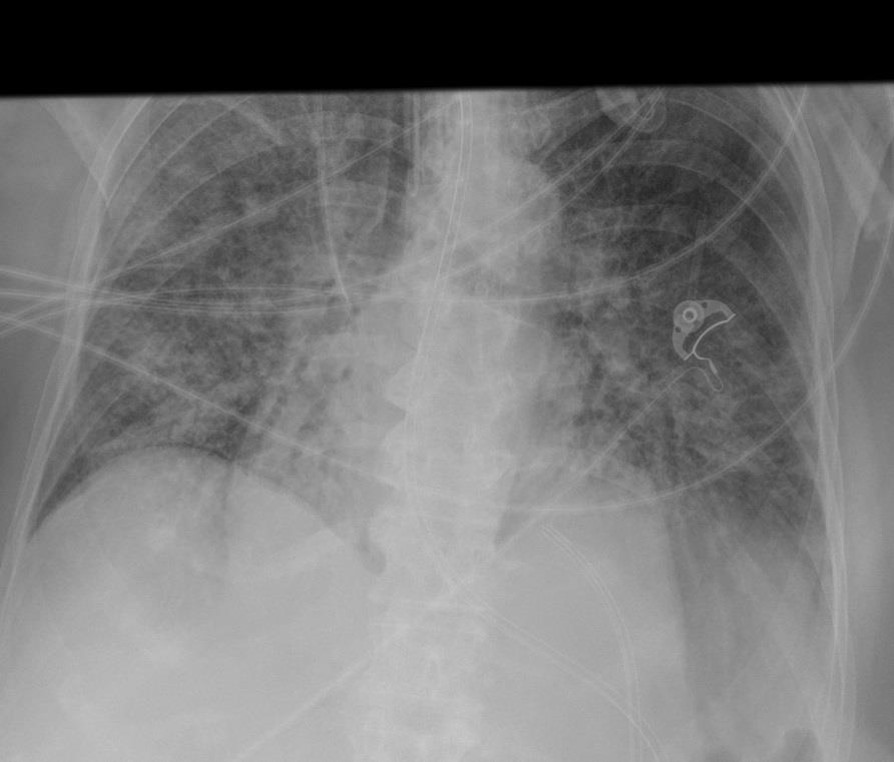
Chest X-ray
- A chest x-ray is done to periodically categorize the severity of ARDS along with arterial blood gases.
- Findings on x-ray suggestive of acute respiratory distress include:
- Cardiomegaly
- Redistribution of vessels
- Peribronchial cuffing
- Pleural effusion
- Septal lines
- Perihilar infiltrates
CT scan
- There are no CT scan findings associated with respiratory failure due to COVID-19.
- However, a CT scan may be helpful in the diagnosis of complications of coronavirus which include the Ground glass (GGO) pattern which is a common finding in COVID-19 patients with following characteristics:
- Multifocal (GGO presents as a unifocal lesion in the early phase of the disease).
- Bilateral
- Peripheral
- Most commonly located in the inferior lobe of the right lung.
MRI
- There are no MRI findings associated with respiratory failure due to COVID-19.
Other Imaging Findings
- There are no other imaging findings associated with respiratory failure due to COVID-19.
Other Diagnostic Studies
- There are no other diagnostic studies associated with respiratory failure due to COVID-19.
Treatment
Medical Therapy
- There is no treatment for respiratory failure due to COVID-19; the mainstay of therapy is supportive care and mechanical ventilation.
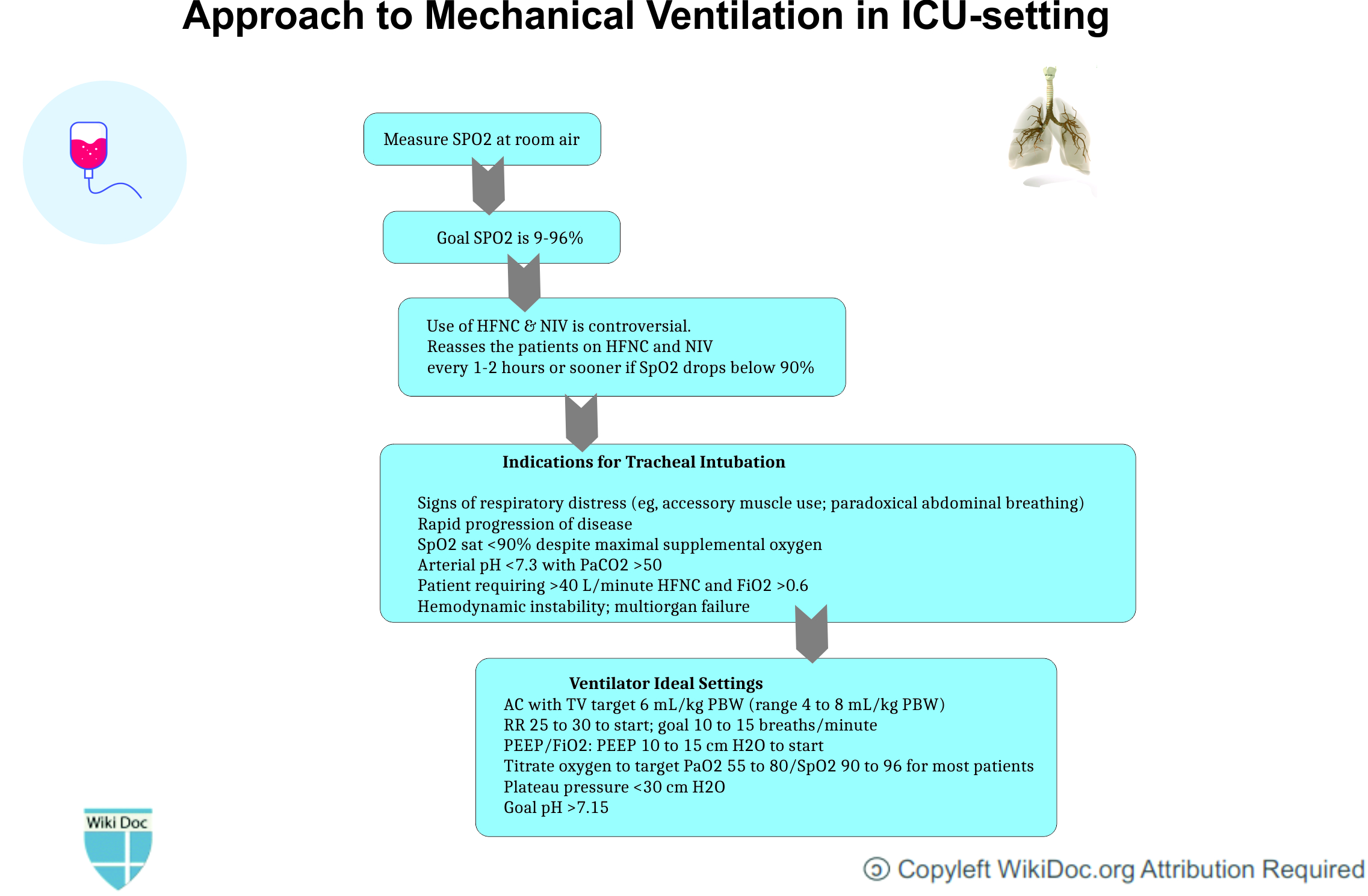
Indications of Tracheal intubation and Mechanical Ventilation
- Following is the list of indications for tracheal intubation and mechanical ventilation in COVID-19 associated respiratory failure:[15]
- Signs of respiratory distress such as:
- Accessory muscle use
- Paradoxical abdominal breathing)
- Rapid progression of the disease
- SpO2 sat <90% despite maximal supplemental oxygen
- Arterial pH <7.3 with PaCO2 >50
- Patient requiring >40 L/minute HFNC and FiO2 >0.6
- Hemodynamic instability; multiorgan failure
- Signs of respiratory distress such as:
Ventilator settings
- Following is the list of ventilator settings required in COVID-19 associated respiratory failure:[16]
| Low Tidal Volume Ventilation is recommended |
|---|
|
Management of Complications
- Daily laboratory studies should be done to monitor for complications including acute kidney injury, pericarditis, sudden cardiac death and superinfection.
- Complete blood count with differential, chemistries, liver function and coagulation studies, arterial blood gases, ferritin level, D-dimer level, and lactate dehydrogenase are usually performed every day.
RECOVERY Trial
- It has been recommended in RECOVERY Trial that for patients requiring mechanical ventilation or those requiring oxygen should be given low dose dexamethasone (6mg daily for 10 days) for ICU patients.[17]
- It has been shown to reduce the mortality rate by 1/3 rd in mechanically ventilated patients.[18]
Prevention
Primary Prevention
- The best way to prevent being infected by COVID-19 is to avoid being exposed to this virus by adopting the following practices for infection control:
- Often wash hands with soap and water for at least 20 seconds.
- Use an alcohol-based hand sanitizer containing at least 60% alcohol in case soap and water are not available.
- Avoid touching the eyes, nose, and mouth without washing hands.
- Avoid being in close contact with people sick with COVID-19 infection.
- Stay home while being symptomatic to prevent spread to others.
- Cover mouth while coughing or sneezing with a tissue paper, and then throw the tissue in the trash.
- Clean and disinfect the objects and surfaces which are touched frequently.
- There is currently no vaccine available to prevent COVID-19.
Secondary Prevention
- The secondary prevention measures of Coronavirus disease 2019 (COVID-19) constitute protective measures to make sure that an infected individual does not transfer the disease to others by maintaining self-isolation at home or designated quarantine facilities.
References
- ↑ 1.0 1.1 1.2 Li, Xu; Ma, Xiaochun (2020-05-06). "Acute respiratory failure in COVID-19: is it "typical" ARDS?". Critical Care. Springer Science and Business Media LLC. 24 (1). doi:10.1186/s13054-020-02911-9. ISSN 1364-8535.
- ↑ Chen, Yun; Guo, Yao; Pan, Yihang; Zhao, Zhizhuang Joe (2020). "Structure analysis of the receptor binding of 2019-nCoV". Biochemical and Biophysical Research Communications. Elsevier BV. 525 (1): 135–140. doi:10.1016/j.bbrc.2020.02.071. ISSN 0006-291X.
- ↑ 3.0 3.1 Mason, Robert J. (2020). "Pathogenesis of COVID-19 from a cell biology perspective". European Respiratory Journal. European Respiratory Society (ERS). 55 (4): 2000607. doi:10.1183/13993003.00607-2020. ISSN 0903-1936.
- ↑ Zucker, AR (1988). "Therapeutic strategies for acute hypoxemic respiratory failure". Critical care clinics. 4 (4): 813–30. ISSN 0749-0704. PMID 3052710.
- ↑ "Acute Respiratory Distress Syndrome". JAMA. American Medical Association (AMA). 307 (23). 2012-06-20. doi:10.1001/jama.2012.5669. ISSN 0098-7484.
- ↑ Phua, Jason; Weng, Li; Ling, Lowell; Egi, Moritoki; Lim, Chae-Man; Divatia, Jigeeshu Vasishtha; Shrestha, Babu Raja; Arabi, Yaseen M; Ng, Jensen; Gomersall, Charles D; Nishimura, Masaji; Koh, Younsuck; Du, Bin (2020). "Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations". The Lancet Respiratory Medicine. Elsevier BV. 8 (5): 506–517. doi:10.1016/s2213-2600(20)30161-2. ISSN 2213-2600.
- ↑ "Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology". American Journal of Respiratory and Critical Care Medicine. 2020-07-01. Retrieved 2020-07-02.
- ↑ Liu, Kui; Fang, Yuan-Yuan; Deng, Yan; Liu, Wei; Wang, Mei-Fang; Ma, Jing-Ping; Xiao, Wei; Wang, Ying-Nan; Zhong, Min-Hua; Li, Cheng-Hong; Li, Guang-Cai; Liu, Hui-Guo (2020-05-05). "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese medical journal. Ovid Technologies (Wolters Kluwer Health). 133 (9): 1025–1031. doi:10.1097/cm9.0000000000000744. ISSN 0366-6999. PMC 7147277 Check
|pmc=value (help). PMID 32044814 Check|pmid=value (help). - ↑ Arentz, Matt; Yim, Eric; Klaff, Lindy; Lokhandwala, Sharukh; Riedo, Francis X.; Chong, Maria; Lee, Melissa (2020-04-28). "Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State". JAMA. American Medical Association (AMA). 323 (16): 1612. doi:10.1001/jama.2020.4326. ISSN 0098-7484. PMC 7082763 Check
|pmc=value (help). PMID 32191259 Check|pmid=value (help). - ↑ Wu, Chaomin; Chen, Xiaoyan; Cai, Yanping; Xia, Jia’an; Zhou, Xing; Xu, Sha; Huang, Hanping; Zhang, Li; Zhou, Xia; Du, Chunling; Zhang, Yuye; Song, Juan; Wang, Sijiao; Chao, Yencheng; Yang, Zeyong; Xu, Jie; Zhou, Xin; Chen, Dechang; Xiong, Weining; Xu, Lei; Zhou, Feng; Jiang, Jinjun; Bai, Chunxue; Zheng, Junhua; Song, Yuanlin (2020-03-13). "Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China". JAMA internal medicine. American Medical Association (AMA). doi:10.1001/jamainternmed.2020.0994. ISSN 2168-6106. PMC 7070509 Check
|pmc=value (help). PMID 32167524 Check|pmid=value (help). - ↑ Chen, Nanshan; Zhou, Min; Dong, Xuan; Qu, Jieming; Gong, Fengyun; Han, Yang; Qiu, Yang; Wang, Jingli; Liu, Ying; Wei, Yuan; Xia, Jia'an; Yu, Ting; Zhang, Xinxin; Zhang, Li (2020). "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". The Lancet. Elsevier BV. 395 (10223): 507–513. doi:10.1016/s0140-6736(20)30211-7. ISSN 0140-6736.
- ↑ 12.0 12.1 Covelli HD, Nessan VJ, Tuttle WK (1983). "Oxygen derived variables in acute respiratory failure". Crit. Care Med. 11 (8): 646–9. PMID 6409506.
- ↑ 13.0 13.1 El-Khatib MF, Jamaleddine GW (2004). "A new oxygenation index for reflecting intrapulmonary shunting in patients undergoing open-heart surgery". Chest. 125 (2): 592–6. PMID 14769743.
- ↑ 14.0 14.1 Cane RD, Shapiro BA, Templin R, Walther K (1988). "Unreliability of oxygen tension-based indices in reflecting intrapulmonary shunting in critically ill patients". Crit. Care Med. 16 (12): 1243–5. PMID 3191742.
- ↑ Alanazi, Abdullah (2015). "Intubations and airway management: An overview of Hassles through third millennium". Journal of Emergencies, Trauma, and Shock. Medknow. 8 (2): 99. doi:10.4103/0974-2700.145401. ISSN 0974-2700.
- ↑ "About". NHLBI ARDS Network (in italiano). Retrieved 2020-07-02.
- ↑ RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL; et al. (2020). "Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report". N Engl J Med. doi:10.1056/NEJMoa2021436. PMID 32678530 Check
|pmid=value (help). - ↑ "Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19". RECOVERY Trial. 2020-06-16. Retrieved 2020-07-02.