Almotriptan labels and packages
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Labels and Packages
The brands listed are the trademarks of their respective owners and are not the trademarks of Janssen Pharmaceuticals, Inc.
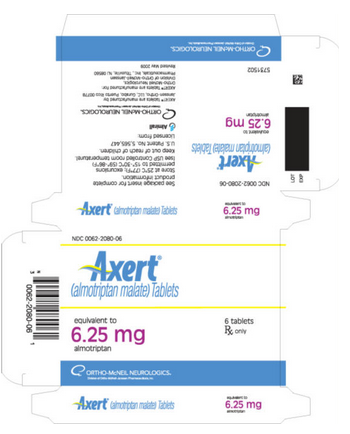
PRINCIPAL DISPLAY PANEL - 6.25 mg Tablet Carton
NDC 50458-211-01
Axert® (almotriptan malate) Tablets
equivalent to 6.25 mg almotriptan
6 tablets
Rx only
Janssen
 |
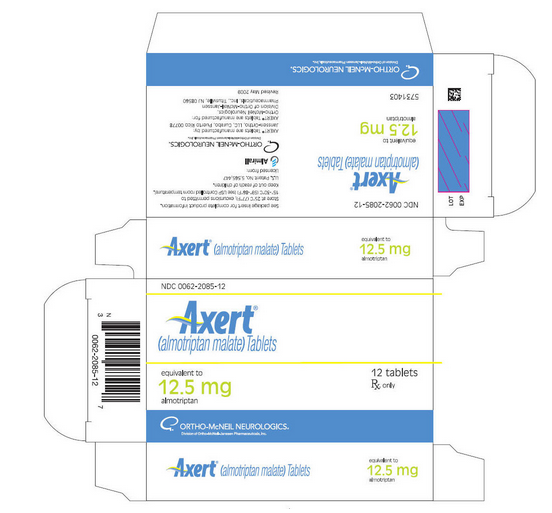
PRINCIPAL DISPLAY PANEL - 12.5 mg Tablet Carton
NDC 50458-210-01
Axert® (almotriptan malate) Tablets
equivalent to 12.5 mg almotriptan
12 tablets
Rx only
Janssen
 |