Adapalene and Benzoyl peroxide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Adapalene and Benzoyl peroxide is a dermatologic agent that is FDA approved for the treatment of acne vulgaris. Common adverse reactions include application site irritation, contact dermatitis, dry skin, erythema, skin irritation, stinging of skin.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acne vulgaris
- EPIDUO gel is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
- Dosing information
- For topical use only; EPIDUO gel is not for oral, ophthalmic, or intravaginal use.
- Apply a thin film of EPIDUO gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes.
Dosage forms and strengths
- Each gram of EPIDUO gel contains 1 mg (0.1%) adapalene and 25 mg (2.5%) benzoyl peroxide in a white to very pale yellow, opaque, aqueous based gel.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Adapalene and Benzoyl peroxide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Adapalene and Benzoyl peroxide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acne vulgaris
- EPIDUO gel is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
- Dosing information
- Apply a thin film of EPIDUO gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Adapalene and Benzoyl peroxide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Adapalene and Benzoyl peroxide in pediatric patients.
Contraindications
- None
Warnings
- 5.1 Ultraviolet Light and Environmental Exposure
Exposure to sunlight, including sunlamps, should be minimized during the use of EPIDUO gel. Patients with high levels of sun exposure and those with inherent sensitivity to sun should exercise particular caution. Use of sunscreen products and protective apparel, (e.g., hat) are recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, may be irritating to patients under treatment with EPIDUO gel.
5.2 Local Cutaneous Reactions
Erythema, scaling, dryness, and stinging/burning may be experienced with use of EPIDUO gel. These are most likely to occur during the first four weeks of treatment, are mostly mild to moderate in intensity, and usually lessen with continued use of the medication. Irritant and allergic contact dermatitis may occur. Depending upon the severity of these adverse reactions, patients should be instructed to use a moisturizer, reduce the frequency of the application of EPIDUO gel, or discontinue use. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of "waxing" as a depilatory method should be avoided on skin treated with EPIDUO gel.
Avoid concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have strong skin-drying effect and products with high concentrations of alcohol, astringents, spices, or limes).
Adverse Reactions
Clinical Trials Experience
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
During clinical trials, 1401 subjects were exposed to EPIDUO gel. A total of 1036 subjects with acne vulgaris, 12 years and older, were treated once daily for 12 weeks to 12 months. Related adverse events reported within 12 weeks of treatment and in at least 1% of subjects treated with EPIDUO gel and those reported in subjects treated with the vehicle gel are presented in Table 1:

Local tolerability evaluations, presented in Table 2, were conducted at each study visit in clinical trials by assessment of erythema, scaling, dryness, burning, and stinging.
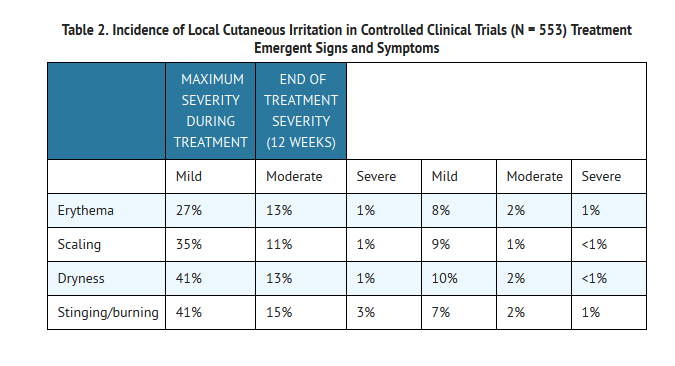
Analysis over the 12-week period showed that local tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 of therapy and decreased thereafter.
During a pediatric clinical trial, 285 children with acne vulgaris, 9 to 11 years of age were treated with EPIDUO gel or with the vehicle gel once daily for 12 weeks. Overall, the safety profile of EPIDUO gel in these subjects is comparable to the safety profile observed in older subjects 12 years of age and above, both in the nature and frequency of the observed adverse events.
Analysis of local tolerability evaluations shows similar incidence of treatment emergent signs and symptoms as in subjects 12 years of age and above, with local tolerability signs and symptoms peaking during the first week and decreasing over time.
Postmarketing Experience
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of EPIDUO gel: eyelid edema, sunburn, blister, pain of skin, pruritus, swelling face, conjunctivitis, skin discoloration, rash, eczema, throat tightness and allergic contact dermatitis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents.
No formal drug-drug interaction studies were conducted with EPIDUO gel.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Pregnancy Category C. There are no well-controlled trials in pregnant women treated with EPIDUO gel. Animal reproduction studies have not been conducted with the combination gel or benzoyl peroxide. Furthermore, such studies are not always predictive of human response; therefore, EPIDUO gel should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
No teratogenic effects were observed in rats treated with oral doses of 0.15 to 5.0 mg adapalene/kg/day, up to 25 times (mg/m2/day) the maximum recommended human dose (MRHD) of 2 grams of EPIDUO gel. However, teratogenic changes were observed in rats and rabbits when treated with oral doses of ≥ 25 mg adapalene/kg/day representing 123 and 246 times MRHD, respectively. Findings included cleft palate, microphthalmia, encephalocele and skeletal abnormalities in rats; and umbilical hernia, exophthalmos and kidney and skeletal abnormalities in rabbits.
Dermal teratology studies conducted in rats and rabbits at doses of 0.6-6.0 mg adapalene/kg/day [25-59 times (mg/m2) the MRHD] exhibited no fetotoxicity and only minimal increases in supernumerary ribs in both species and delayed ossification in rabbits.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Adapalene and Benzoyl peroxide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Adapalene and Benzoyl peroxide during labor and delivery.
Nursing Mothers
It is not known whether adapalene or benzoyl peroxide is excreted in human milk following use of EPIDUO gel. Because many drugs are excreted in human milk, caution should be exercised when EPIDUO gel is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of EPIDUO gel in pediatric patients under the age of 9 have not been established.
Geriatic Use
Clinical studies of EPIDUO gel did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Adapalene and Benzoyl peroxide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Adapalene and Benzoyl peroxide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Adapalene and Benzoyl peroxide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Adapalene and Benzoyl peroxide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Adapalene and Benzoyl peroxide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Adapalene and Benzoyl peroxide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Adapalene and Benzoyl peroxide Administration in the drug label.
Monitoring
There is limited information regarding Adapalene and Benzoyl peroxide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Adapalene and Benzoyl peroxide and IV administrations.
Overdosage
There is limited information regarding Adapalene and Benzoyl peroxide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Adapalene and Benzoyl peroxide Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Adapalene and Benzoyl peroxide Mechanism of Action in the drug label.
Structure
- EPIDUO (adapalene and benzoyl peroxide) gel, 0.1%/2.5% is a white to very pale yellow, opaque gel for topical use containing adapalene 0.1% and benzoyl peroxide 2.5%.
- Active: Adapalene, a synthetic retinoid, is a naphthoic acid derivative with retinoid-like properties. The chemical name for adapalene is (6-[3-(1-adamantyl)-4-methoxyphenyl]-2- naphthoic acid). It has the following structural formula:

- Molecular formula: C28H28O3 Molecular weight: 412.5
- Benzoyl Peroxide is a highly lipophilic oxidizing agent that localizes in both bacterial and keratinocyte cell membranes. The chemical name for benzoyl peroxide is dibenzoyl peroxide. It has the following structural formula:

- Molecular formula: C14H10O4 Molecular weight: 242.23
- EPIDUO gel contains the following inactive ingredients: acrylamide/sodium acryloyldimethyltaurate copolymer, docusate sodium, edetate disodium, glycerin, isohexadecane, poloxamer 124, polysorbate 80, propylene glycol, purified water, and sorbitan oleate.
Pharmacodynamics
There is limited information regarding Adapalene and Benzoyl peroxide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Adapalene and Benzoyl peroxide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Adapalene and Benzoyl peroxide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Adapalene and Benzoyl peroxide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Adapalene and Benzoyl peroxide How Supplied in the drug label.
Storage
There is limited information regarding Adapalene and Benzoyl peroxide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Adapalene and Benzoyl peroxide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Adapalene and Benzoyl peroxide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Adapalene and Benzoyl peroxide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Adapalene and Benzoyl peroxide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Epiduo
Look-Alike Drug Names
There is limited information regarding Adapalene and Benzoyl peroxide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.