Acyclovir (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acyclovir (oral) is a antiviral agent that is FDA approved for the treatment of herpes zoster infections, genital herpes, chickenpox. Common adverse reactions include nausea, vomiting, diarrhea, malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Herpes Zoster Infections
- Acyclovir is indicated for the acute treatment of herpes zoster (shingles).
- Dosing Information
- (Dosage)
Genital Herpes
- Acyclovir is indicated for the treatment of initial episodes and the management of recurrent episodes of genital herpes.
- Dosing Information
- (Dosage)
Chickenpox
- Acyclovir is indicated for the treatment of chickenpox (varicella).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
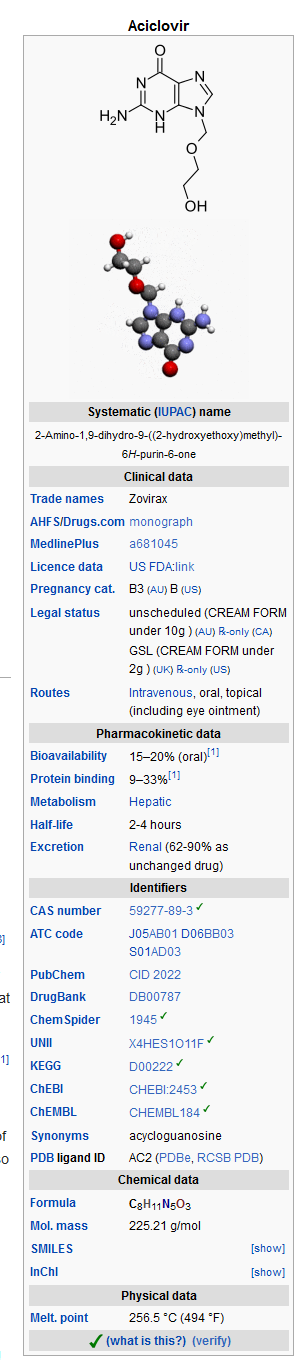
Acyclovir (oral)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Mechanism of Antiviral Action
- Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV- 2), and varicella-zoster virus (VZV).
- The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities
- The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC50 of 1.35 mcg/mL.
Drug Resistance
- Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
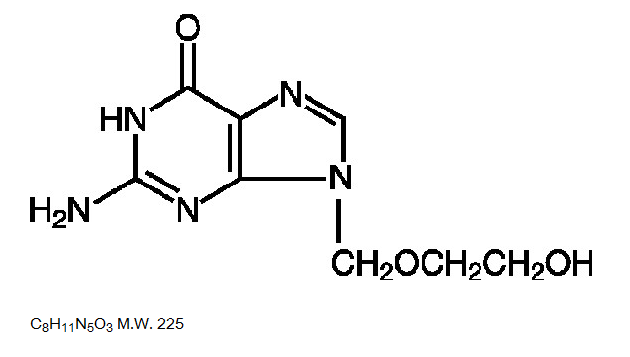
Structure
- Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Each capsule, for oral administration, contains 200 mg of acyclovir. In addition, each capsule contains the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate and sodium lauryl sulfate. The capsule shell consists of gelatin, FD&C blue No. 1, D&C red No. 28, D&C red No. 33 and titanium dioxide. Printed with edible black ink that contains FD&C blue No. 1, FD&C blue No. 2, FD&C red No. 40 and D&C yellow No. 10. Each tablet, for oral administration, contains 400 mg or 800 mg of acyclovir. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch and sodium starch glycolate. The 400 mg tablets also contain FD&C blue No. 2.
- Acyclovir is a white to off-white, crystalline powder. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
- The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
Pharmacodynamics
(Description)
Pharmacokinetics
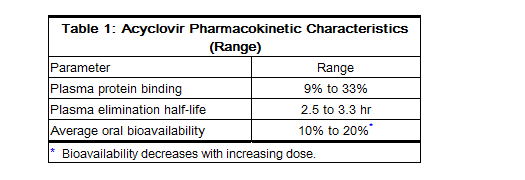
- The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
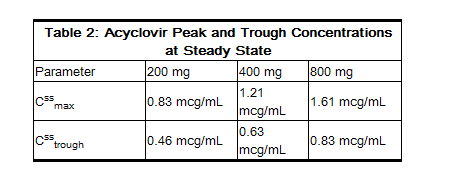
- In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
- There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir capsules and tablets may be administered with or without food.
- The only known urinary metabolite is 9-[(carboxymethoxy)methyl]guanine.
Special Populations
Adults With Impaired Renal Function
- The half-life and total body clearance of acyclovir are dependent on renal function. A dosage adjustment is recommended for patients with reduced renal function (see DOSAGE AND ADMINISTRATION).
Geriatrics
- Acyclovir plasma concentrations are higher in geriatric patients compared to younger adults, in part due to age-related changes in renal function. Dosage reduction may be required in geriatric patients with underlying renal impairment (see PRECAUTIONS, Geriatric Use).
Pediatrics
- In general, the pharmacokinetics of acyclovir in pediatric patients is similar to that of adults. Mean half-life after oral doses of 300 mg/m2 and 600 mg/m2 in pediatric patients aged 7 months to 7 years was 2.6 hours (range 1.59 to 3.74 hours).
Drug Interactions
- Coadministration of probenecid with intravenous acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
Clinical Trials
Initial Genital Herpes
- Double-blind, placebo-controlled studies have demonstrated that orally administered acyclovir significantly reduced the duration of acute infection and duration of lesion healing. The duration of pain and new lesion formation was decreased in some patient groups.
Recurrent Genital Herpes
- Double-blind, placebo-controlled studies in patients with frequent recurrences (6 or more episodes per year) have shown that orally administered acyclovir given daily for 4 months to 10 years prevented or reduced the frequency and/or severity of recurrences in greater than 95% of patients.
- In a study of patients who received acyclovir 400 mg twice daily for 3 years, 45%, 52%, and 63% of patients remained free of recurrences in the first, second, and third years, respectively. Serial analyses of the 3 month recurrence rates for the patients showed that 71% to 87% were recurrence free in each quarter.
Herpes Zoster Infections
- In a double-blind, placebo-controlled study of immunocompetent patients with localized cutaneous zoster infection, acyclovir (800 mg 5 times daily for 10 days) shortened the times to lesion scabbing, healing, and complete cessation of pain, and reduced the duration of viral shedding and the duration of new lesion formation.
- In a similar double-blind, placebo-controlled study, acyclovir (800 mg 5 times daily for 7 days) shortened the times to complete lesion scabbing, healing, and cessation of pain; reduced the duration of new lesion formation; and reduced the prevalence of localized zoster-associated neurologic symptoms (paresthesia, dysesthesia, or hyperesthesia).
- Treatment was begun within 72 hours of rash onset and was most effective if started within the first 48 hours.
Adults greater than 50 years of age showed greater benefit.
Chickenpox
- Three randomized, double-blind, placebo-controlled trials were conducted in 993 pediatric patients aged 2 to 18 years with chickenpox. All patients were treated within 24 hours after the onset of rash. In 2 trials, acyclovir was administered at 20 mg/kg 4 times daily (up to 3,200 mg per day) for 5 days. In the third trial, doses of 10, 15, or 20 mg/kg were administered 4 times daily for 5 to 7 days. Treatment with acyclovir shortened the time to 50% healing; reduced the maximum number of lesions; reduced the median number of vesicles; decreased the median number of residual lesions on day 28; and decreased the proportion of patients with fever, anorexia, and lethargy by day 2. Treatment with acyclovir did not affect varicella-zoster virus-specific humoral or cellular immune responses at 1 month or 1 year following treatment.
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Acyclovir (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Acyclovir (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Acyclovir (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Acyclovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Acyclovir (oral) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938947.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N947;800 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938947
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780253.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=M;253 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780253
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780302.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=MYLAN;302 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780302
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ACYCLOVIR_NDC_09045789.jpg |Drug Name=ACYCLOVIR |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=APO;042 |Pill Dosage=200 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09045789
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910108.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910108
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910109.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910109
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290711.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;A05 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290711
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290712.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290712
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290713.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290713
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290911.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;CTI;111; |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290911
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=acyclovir_NDC_605055306.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5306 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055306
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420111.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=200;CTI;111 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420111
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040505.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX505 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040505
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040652.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX652 |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040652
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_672530101.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=A02;400 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=DAVA Pharmaceuticals, Inc. |NDC=672530101
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938940.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N940;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938940
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938943.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N943;400 |Pill Dosage=400 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938943
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=acyclovir_NDC_605055307.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5307 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055307
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420112.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420112
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040504.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX504 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040504
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730945.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX;800 |Pill Dosage=800 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730945
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730949.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Hexagon |Pill Size (mm)=12 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730949
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730991.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Wellcome;ZOVIRAX;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730991
}}