Acyclovir (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acyclovir (oral) is a antiviral agent that is FDA approved for the treatment of herpes zoster infections, genital herpes, chickenpox. Common adverse reactions include nausea, vomiting, diarrhea, malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Acyclovir (oral)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Mechanism of Antiviral Action
- Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV- 2), and varicella-zoster virus (VZV).
- The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities
- The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC50 of 1.35 mcg/mL.
Drug Resistance
- Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
Structure
- Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Each capsule, for oral administration, contains 200 mg of acyclovir. In addition, each capsule contains the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate and sodium lauryl sulfate. The capsule shell consists of gelatin, FD&C blue No. 1, D&C red No. 28, D&C red No. 33 and titanium dioxide. Printed with edible black ink that contains FD&C blue No. 1, FD&C blue No. 2, FD&C red No. 40 and D&C yellow No. 10. Each tablet, for oral administration, contains 400 mg or 800 mg of acyclovir. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch and sodium starch glycolate. The 400 mg tablets also contain FD&C blue No. 2.
- Acyclovir is a white to off-white, crystalline powder. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
- The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
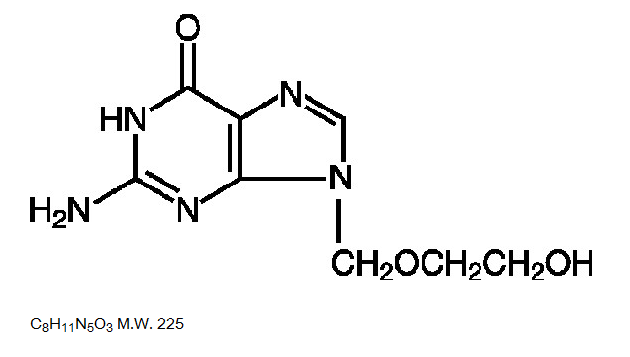
Pharmacodynamics
(Description)
Pharmacokinetics
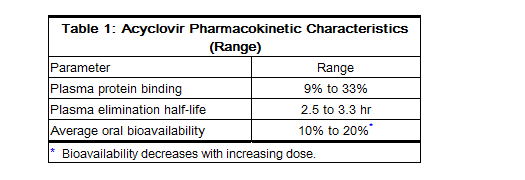
- The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
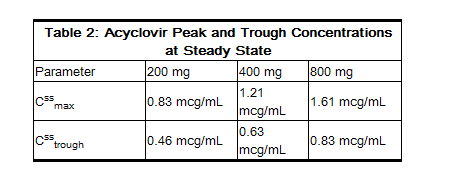
- In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
- There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir capsules and tablets may be administered with or without food.
- The only known urinary metabolite is 9-[(carboxymethoxy)methyl]guanine.
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Acyclovir (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Acyclovir (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Acyclovir (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Acyclovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Acyclovir (oral) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938947.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N947;800 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938947
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780253.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=M;253 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780253
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780302.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=MYLAN;302 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780302
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ACYCLOVIR_NDC_09045789.jpg |Drug Name=ACYCLOVIR |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=APO;042 |Pill Dosage=200 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09045789
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910108.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910108
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910109.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910109
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290711.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;A05 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290711
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290712.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290712
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290713.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290713
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290911.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;CTI;111; |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290911
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=acyclovir_NDC_605055306.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5306 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055306
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420111.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=200;CTI;111 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420111
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040505.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX505 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040505
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040652.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX652 |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040652
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_672530101.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=A02;400 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=DAVA Pharmaceuticals, Inc. |NDC=672530101
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938940.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N940;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938940
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938943.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N943;400 |Pill Dosage=400 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938943
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=acyclovir_NDC_605055307.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5307 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055307
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420112.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420112
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040504.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX504 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040504
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730945.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX;800 |Pill Dosage=800 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730945
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730949.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Hexagon |Pill Size (mm)=12 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730949
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730991.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Wellcome;ZOVIRAX;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730991
}}