Clevidipine description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description
Cleviprex is a sterile, milky-white emulsion containing 0.5 mg/mL of clevidipine suitable for intravenous administration. Clevidipine is a dihydropyridine calcium channel blocker. Chemically, the active substance, clevidipine, is butyroxymethyl methyl 4-(2´,3´-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate. It is a racemic mixture with a molecular weight of 456.3 g/mol. Each enantiomer has equipotent antihypertensive activity. The structure and formula are:
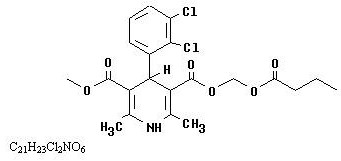 |
 |
Clevidipine is practically insoluble in water and is formulated in an oil-in-water emulsion. In addition to the active ingredient, clevidipine, Cleviprex contains soybean oil (200 mg/mL), glycerin (22.5 mg/mL), purified egg yolk phospholipids (12 mg/mL), oleic acid (0.3 mg/mL), disodium edetate (0.05 mg/mL), and sodium hydroxide to adjust pH. Cleviprex has a pH of 6.0 – 8.0 and is a ready-to-use emulsion. [1]
References
- ↑ "CLEVIPREX (CLEVIDIPINE) EMULSION [THE MEDICINES COMPANY]". Retrieved 27 February 2014.