Lopressor description
 | |
| Clinical data | |
|---|---|
| Trade names | Lopressor, Toprol-xl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Elimination half-life | 3-7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C15H25NO3 |
| Molar mass | 267.364 g/mol |
| 3D model (JSmol) | |
| Melting point | 120 °C (248 °F) |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
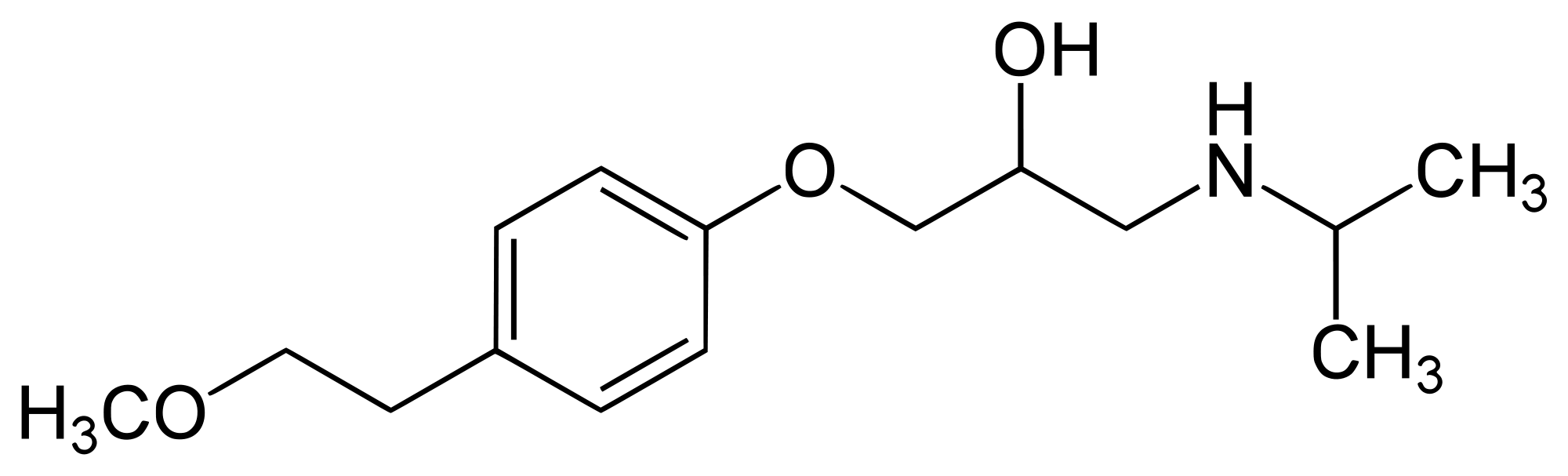
Lopressor, metoprolol tartrate USP, is a selective beta1-adrenoreceptor blocking agent, available as 50- and 100-mg tablets for oral administration and in 5-mL ampuls for intravenous administration. Each ampul contains a sterile solution of metoprolol tartrate USP, 5 mg, and sodium chloride USP, 45 mg, and water for injection USP. Metoprolol tartrate USP is (±)-1-(Isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol L-(+)-tartrate (2:1) salt, and its structural formula is:
 |
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Inactive Ingredients: Tablets contain cellulose compounds, colloidal silicon dioxide, D&C Red No. 30 aluminum lake (50-mg tablets), FD&C Blue No. 2 aluminum lake (100-mg tablets), lactose, magnesium stearate, polyethylene glycol, propylene glycol, povidone, sodium starch glycolate, talc, and titanium dioxide.[1]
References
Adapted from the FDA Package Insert.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Drugs
- Cardiovascular Drugs
- Beta blockers