Azithromycin (ophthalmic) description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite® (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of approximately 290 mOsm/kg.
Preservative: 0.003% benzalkonium chloride. Inactives: mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water for injection, and sodium hydroxide to adjust pH to 6.3.
Azithromycin is a macrolide antibiotic with a 15-membered ring. Its chemical name is (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-aza-cyclopentadecan-15-one, and the structural formula is:
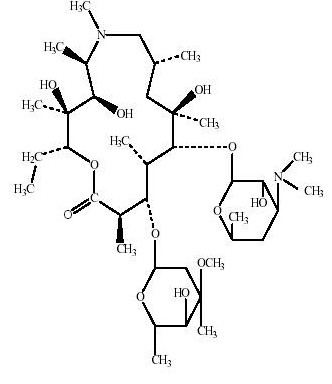 |
Azithromycin has a molecular weight of 749, and its empirical formula is C38H72N2O12.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.