L-glutamine oral powder (Endari)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
L-glutamine oral powder (Endari) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of L-glutamine oral powder (Endari) in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of L-glutamine oral powder (Endari) and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
L-glutamine oral powder (Endari)
| |
| Systematic (IUPAC) name | |
| Glutamine | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 146.15 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | decomposes around 185°C °C (Expression error: Unrecognized word "decomposes". °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral |
Mechanism of Action
- The mechanism of action of the amino acid L-glutamine in treating sickle cell disease (SCD) is not fully understood. Oxidative stress phenomena are involved in the pathophysiology of SCD. Sickle red blood cells (RBCs) are more susceptible to oxidative damage than normal RBCs, which may contribute to the chronic hemolysis and vaso-occlusive events associated with SCD. The pyridine nucleotides, NAD+ and its reduced form NADH, play roles in regulating and preventing oxidative damage in RBCs. L-glutamine may improve the NAD redox potential in sickle RBCs through increasing the availability of reduced glutathione.
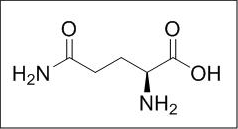
Structure
Pharmacodynamics
- In vivo analyses demonstrated that L-glutamine supplementation improved NAD redox potential.
Pharmacokinetics
- The pharmacokinetics of L-glutamine has been studied in healthy subjects and a variety of disease states. Relevant results from published literature are summarized below.
Absorption
- Following single-dose oral administration of L-glutamine at 0.1 g/kg, mean peak L-glutamine concentration was 1028 µM (or 150 mcg/mL) occurring approximately 30 minutes after administration. The pharmacokinetics following multiple oral doses has not been characterized.
Distribution
- After an intravenous (IV) bolus dose, the volume of distribution was estimated to be approximately 200 mL/kg.
Elimination
- After an intravenous bolus dose, the terminal half-life of L-glutamine was approximately one hour.
Metabolism
- Endogenous L-glutamine participates in various metabolic activities, including the formation of glutamate, and synthesis of proteins, nucleotides, and amino sugars. Exogenous L-glutamine is anticipated to undergo similar metabolism.
Excretion
- Metabolism is the major route of elimination for L-glutamine. Although L-glutamine is eliminated by glomerular filtration, it is almost completely reabsorbed by the renal tubules.
Specific Populations
- The safety of Endari has not been established in patients with renal or hepatic impairment.
Drug Interactions
- No drug interaction studies have been conducted.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of L-glutamine.
- L-glutamine was not mutagenic in a bacterial mutagenicity (Ames) assay, nor clastogenic in a chromosome aberration assay in mammalian (Chinese Hamster Lung CHL/IU) cells.
- Animal reproduction studies and its potential for impairment of fertility have not been conducted with L-glutamine . It is also not known whether L-glutamine can cause fetal harm when administered to a pregnant woman or whether it can affect reproductive capacity.
Clinical Studies
- The efficacy of L-glutamine oral powder in sickle cell disease was evaluated in a randomized, double-blind, placebo-controlled, multi-center clinical trial entitled "A Phase III Safety and Efficacy Study of L-Glutamine to Treat Sickle Cell Disease or Sickle βo-thalassemia" [NCT01179217] (see TABLE 3).
- The clinical trial evaluated the efficacy and safety of L-glutamine oral powder in 230 patients (5 to 58 years of age) with sickle cell anemia or sickle β0-thalassemia who had 2 or more painful crises within 12 months prior to enrollment. Eligible patients stabilized on hydroxyurea for at least 3 months continued their therapy throughout the study. The trial excluded patients who had received blood products within 3 weeks, had renal insufficiency or uncontrolled liver disease, or were pregnant (or planning pregnancy) or lactating. Study patients received Endari or placebo for a treatment duration of 48 weeks followed by 3 weeks of tapering.
- Efficacy was demonstrated by a reduction in the number of sickle cell crises through Week 48 and prior to the start of tapering among patients that received Endari compared to patients who received placebo. A sickle cell crisis was defined as a visit to an emergency room/medical facility for sickle cell disease-related pain which was treated with a parenterally administered narcotic or parenterally administered ketorolac. In addition, the occurrence of chest syndrome, priapism, and splenic sequestration were considered sickle cell crises. Treatment with Endari also resulted in fewer hospitalizations due to sickle cell pain at Week 48, fewer cumulative days in hospital and a lower incidence of acute chest syndrome.

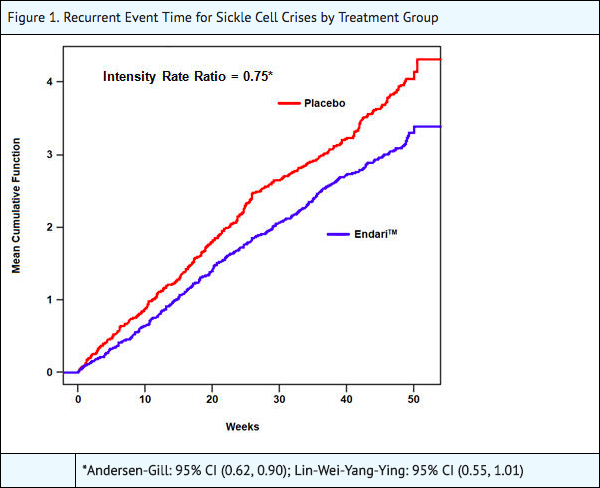
- The recurrent crisis event time analysis (Figure 1) yielded an intensity rate ratio (IRR) value of 0.75 with 95% CI= (0.62, 0.90) and (0.55, 1.01) based on unstratified models using the Andersen-Gill and Lin, Wei, Yang and Ying methods, respectively in favor of Endari, suggesting that over the entire 48-week period, the average cumulative crisis count was reduced by 25% from the Endari group over the placebo group.
How Supplied
- Endari is supplied in paper-foil-plastic laminate packets containing 5 grams of L-glutamine white crystalline powder.
- Carton of 60 packets: NDC 42457-420-60
Storage
- Store at 20°C to 25°C (68°F to 77°F) away from direct sunlight.
Images
Drug Images
{{#ask: Page Name::L-glutamine oral powder (Endari) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::L-glutamine oral powder (Endari) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Dosage and Administration
- Advise patient to take a missed dose as soon as they remember. Patient should not double the dose that they take.
- Instruct patient to mix each dose in 8 oz. (240 mL) of cold or room temperature beverage or 4 to 6 oz. of food.
- Advise patient that complete dissolution is not required prior to administration.
Precautions with Alcohol
Alcohol-L-glutamine oral powder (Endari) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Endari
Look-Alike Drug Names
There is limited information regarding L-glutamine oral powder (Endari) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.