Sandbox Riociguat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
*Do not administer Adempas to a pregnant female because it may cause fetal harm.
|
Overview
Sandbox Riociguat is a Soluble Guanylate Cyclase Stimulator that is FDA approved for the treatment of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) and Pulmonary Arterial Hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include fetal harm (teratogenic effects).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The recommended starting dosage is 1 mg taken 3 times a day. For patients who may not tolerate the hypotensive effect of Riociguat, consider a starting dose of 0.5 mg taken three times a day. If systolic blood pressure remains greater than 95 mmHg and the patient has no signs or symptoms of hypotension, up-titrate the dose by 0.5 mg taken three times a day. Dose increases should be no sooner than 2 weeks apart. The dose can be increased to the highest tolerated dosage, up to a maximum of 2.5 mg taken three times a day. If at any time, the patient has symptoms of hypotension, decrease the dosage by 0.5 mg taken three times a day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sandbox Riociguat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sandbox Riociguat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of Adempas in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sandbox Riociguat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sandbox Riociguat in pediatric patients.
Contraindications
Pregnant Women
- Adempas may cause fetal harm when administered to a pregnant woman. Adempas is contraindicated in females who are pregnant. Adempas was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Nitrates
- Co-administration of Adempas with nitrates or nitric oxide donors (such as amyl nitrite) in any form is contraindicated.
PDE-5 inhibitors
- Concomitant administration of Adempas with specific PDE-5 inhibitors (such as sildenafil, tadalafil, or vardenafil) or nonspecific PDE inhibitors (such as dipyridamole or theophylline) is contraindicated.
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
*Do not administer Adempas to a pregnant female because it may cause fetal harm.
|
Fetal Harm
- Adempas may cause fetal harm when administered during pregnancy and is contraindicated for use in women who are pregnant. In females of reproductive potential, exclude pregnancy prior to initiation of therapy, advise use of acceptable contraception and obtain monthly pregnancy tests.
Low Blood Pressure
- Adempas reduces blood pressure. Consider the potential for symptomatic hypotension or ischemia in patients with hypovolemia, left ventricular outflow tract obstruction, resting hypotension, autonomic dysfunction, or concomitant treatment with antihypertensives or strong CYP and P-gp/BCRP inhibitors
Adverse Reactions
Clinical Trials Experience
The safety data described below reflect exposure to Adempas in two, randomized, double blind, placebo-controlled trials in patients with inoperable or recurrent/persistent CTEPH (CHEST-1) and treatment naive or pre-treated PAH patients (PATENT-1). The population (Adempas: n = 490; Placebo: n = 214) was between the age of 18 and 80 years.
The safety profile of Adempas in patients with inoperable or recurrent/persistent CTEPH (CHEST-1) and treatment naive or pre-treated PAH (PATENT-1) were similar. Therefore, adverse drug reactions (ADRs) identified from the 12 and 16 week placebo-controlled trials for PAH and CTEPH respectively were pooled, and those occurring more frequently on Adempas than placebo (≥3%) are displayed in Table 1 below. Most adverse reactions in Table 1 can be ascribed to the vasodilatory mechanism of action of Adempas.

Postmarketing Experience
No postmarketing experience is currently available because Adempas was only recently approved by the FDA in 2013.
Drug Interactions
Nitrates
- Co-administration of Adempas with nitrates or nitric oxide donors (such as amyl nitrite) in any form is contraindicated because of hypotension.
PDE Inhibitors
- Co-administration of Adempas with specific PDE-5 inhibitors (such as sildenafil, tadalafil, or vardenafil) and nonspecific PDE inhibitors (such as dipyridamole or theophylline), is contraindicated because of hypotension. Clinical experience with co-administration of Adempas and other phosphodiesterase inhibitors (for example, milrinone, cilostazole, roflumilast) is limited.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X
Adempas may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy. Adempas was teratogenic and embryotoxic in rats at doses with exposures to unbound drug that were approximately 8 times and 2 times, respectively, the human exposure. In rabbits, riociguat led to abortions at 4 times the human exposure and fetal toxicity with exposures approximately 13 times the human exposure. If Adempas is used in pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to the fetus.
Pregnancy Category (AUS): D
"While no increase in malformation rate was observed in the rabbit embryofetal development study, exaggerated pharmacological effects of riociguat on the F1 dams at ≥ 1.5 mg/kg/day led to an increased rate of abortion and total resorption. At the maternal and fetal NOAEL of 0.5 mg/kg/day the ERAUCU was similar to that expected clinically.
Riociguat prolonged the mean gestation time and reduced maternal bodyweight gain at the high dose (HD) of 15 mg/kg/day in the pre and postnatal development studies in rats (maternal NOAEL of 5 mg/kg/day, ERAUCU of about 1). A reduced number of live births were observed at doses ≥ 5 mg/kg/day but there were no notable effects on the development of the surviving pups including on their reproductive performance (NOAEL for pup development of 15 mg/kg/day, ERAUCU approximately 2 to 3).
The M1 metabolite BAY 60-4552 was not well tolerated in reproductive studies and the NOAEL for maternal and fetal toxicity was 2 mg/kg/day (ERAUCU <0.1). Doses of 5 mg/kg/day in rabbits resulted in increased rates of abortion. There was, however, no evidence of a direct teratogenic effect in either species." [1]
Labor and Delivery
There are no guidelines for female patients undergoing labor and delivery because Adempas is contraindicated for pregnancy. The patient's risk should be reevaluated after delivery.
Nursing Mothers
It is not known if Adempas is present in human milk. Riociguat or its metabolites were present in the milk of rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from riociguat, either discontinue nursing or Adempas.
Pediatric Use
Safety and effectiveness of Adempas in pediatric patients have not been established. In growing rats, however, effects on bone formation were observed, including thickening of the growth plates, disorganized trabecular bone, and diffuse hyperostosis.
Geriatic Use
Of the total number of subjects in clinical studies of Adempas, 23% were 65 and over, and 6% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
Besides issues with pregnancy, there are no gender-specific guidelines for Adempas.
Race
There are no race-specific guidelines for Adempas.
Renal Impairment
Safety and efficacy have not been demonstrated in patients with creatinine clearance <15 mL/min or on dialysis.
Hepatic Impairment
Safety and efficacy have not been demonstrated in patients with severe hepatic impairment (Child Pugh C).
Females of Reproductive Potential and Males
Pregnancy Testing
- Female patients of reproductive potential must have a negative pregnancy test prior to starting treatment with Adempas, monthly during treatment, and one month after discontinuation of treatment with Adempas. Advise patients to contact their healthcare provider if they become pregnant or suspect they may be pregnant. Counsel patients on the risk to the fetus.
Contraception
- Female patients of reproductive potential must use acceptable methods of contraception during treatment with Adempas and for 1 month after treatment with Adempas. Patients may choose one highly effective form of contraception (intrauterine devices [IUD], contraceptive implants or tubal sterilization) or a combination of methods (hormone method with a barrier method or two barrier methods). If a partner’s vasectomy is the chosen method of contraception, a hormone or barrier method must be used along with this method. Counsel patients on pregnancy planning and prevention, including emergency contraception, or designate counseling by another healthcare provider trained in contraceptive counseling.
Immunocompromised Patients
There are no guidelines for the use of Adempas in immunocompromised patients at this time.
Administration and Monitoring
Administration
Oral.
Monitoring
Monitor for signs and symptoms of hypotension on initiation and on treatment with strong CYP and P-gp/BCRP inhibitors. A dose reduction should be considered in patients who may not tolerate the hypotensive effect of riociguat. Patient should also have blood pressure checked every two weeks to determine if a change in dosage is necessary.
IV Compatibility
There is limited information regarding the compatibility of Adempas and IV administrations.
Overdosage
In cases of overdose, blood pressure should be closely monitored and supported as appropriate. Based on extensive plasma protein binding, riociguat is not expected to be dialyzable.
Pharmacology
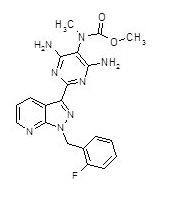
| |
riociguat
| |
| Systematic (IUPAC) name | |
| methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl)carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 422.42 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 247 °C (477 °F) |
| Pharmacokinetic data | |
| Bioavailability | 94% |
| Metabolism | Hepatic via CYP1A1, CYP3A, CYP2C8, CYP2J2 |
| Half life | 12 hours |
| Excretion | Renal (40%) and fecal (53%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Sandbox Riociguat Mechanism of Action in the drug label.
Structure
There is limited information regarding Sandbox Riociguat Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sandbox Riociguat Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sandbox Riociguat Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sandbox Riociguat Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sandbox Riociguat Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sandbox Riociguat How Supplied in the drug label.
Storage
There is limited information regarding Sandbox Riociguat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sandbox Riociguat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sandbox Riociguat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sandbox Riociguat Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sandbox Riociguat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sandbox Riociguat Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sandbox Riociguat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Australian Government Department of Health. (2014, June). Australian Public Assessment Report for Riociguat (Bayer Australia, Ltd., Comp.).