Nafarelin
For patient information, click here.
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Nasal spray |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 2.6 to 4 hours |
| Excretion | renal |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
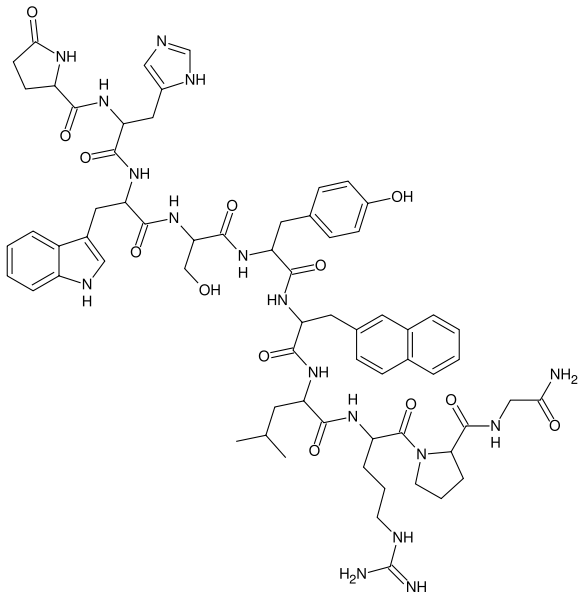
| Formula | C66H83N17O13 |
| Molar mass | 1321.6344 g/mol |
|
WikiDoc Resources for Nafarelin |
|
Articles |
|---|
|
Most recent articles on Nafarelin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Nafarelin at Clinical Trials.gov Clinical Trials on Nafarelin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Nafarelin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Nafarelin Discussion groups on Nafarelin Directions to Hospitals Treating Nafarelin Risk calculators and risk factors for Nafarelin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Nafarelin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Nafarelin is a gonadotropin-releasing hormone agonist (GnRH agonist). By causing constant stimulation of the pituitary, it decreases pituitary secretion of gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH). Nafarelin may be used in the treatment of estrogen-dependent conditions (such as endometriosis or uterine fibroids), to treat central precocious puberty, and to control ovarian stimulation in IVF.
It is normally delivered via a nasal spray.
Nafarelin acetate is marketed by Searle under the brand name Synarel.
Side effects
Side effects of nafarelin are mostly related to the low estrogen state. Side effects include hot flashes, vaginal dryness, headaches, mood changes, and decreased interest in sex. Some patients may experience acne, muscle pain, reduced breast size, and irritation of the tissue inside the nose. These side effects should disappear after stopping the medication.
Template:SIB Template:Sex hormones Template:Pituitary and hypothalamic hormones and analogues
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- GnRH agonists
- Drugs
- Endocrinology