Thrombophilia medical therapy
|
Thrombophilia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Thrombophilia medical therapy On the Web |
|
American Roentgen Ray Society Images of Thrombophilia medical therapy |
|
Risk calculators and risk factors for Thrombophilia medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Asiri Ediriwickrema, M.D., M.H.S. [2] Jaspinder Kaur, MBBS[3]
Overview
The treatment for thrombophilia depends on the underlying hypercoagulable state and the clinical presentation.[1][2][3] The mainstay of therapy for thrombophilia is anticoagulation with either warfarin, low molecular weight heparin, direct Xa inhibitors, or direct thrombin inhibitors.[4][5][6] Treatment should be tailored to the individual patient. The risks and benefits, required monitoring, and costs associated with each form of anticoagulation should be discussed with the patient prior to initiation of therapy. All patients on anticoagulation should be monitored for bleeding.
Medical Therapy
- Treatment algorithims for both acquired and inherited thrombophilias are presented below:
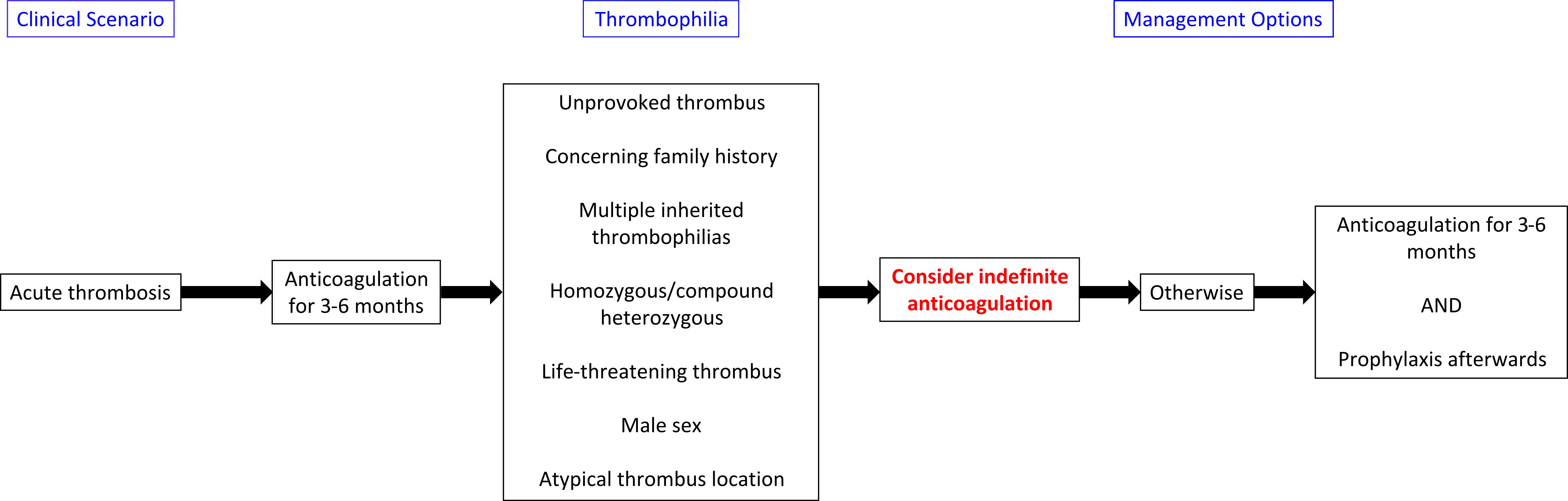
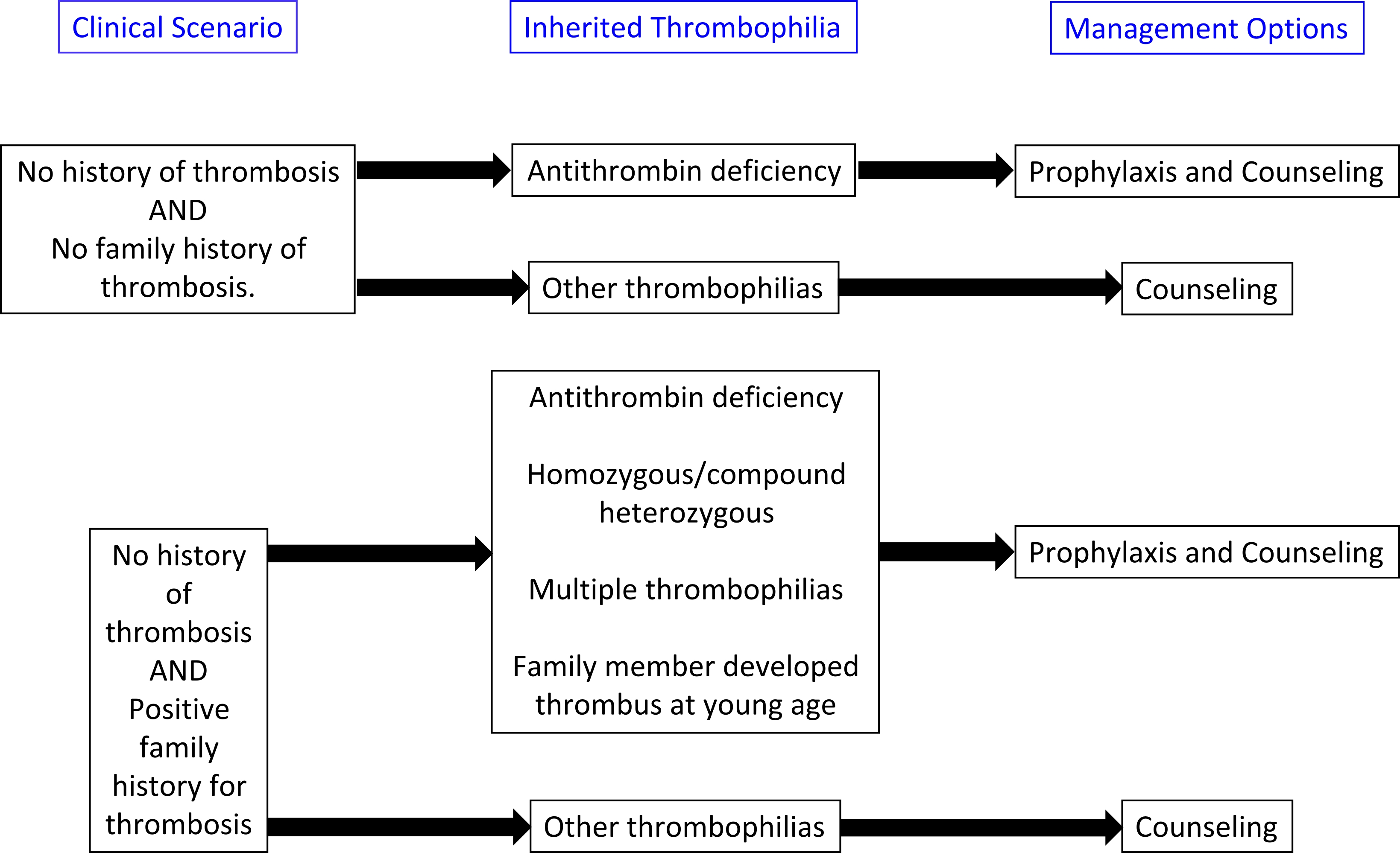
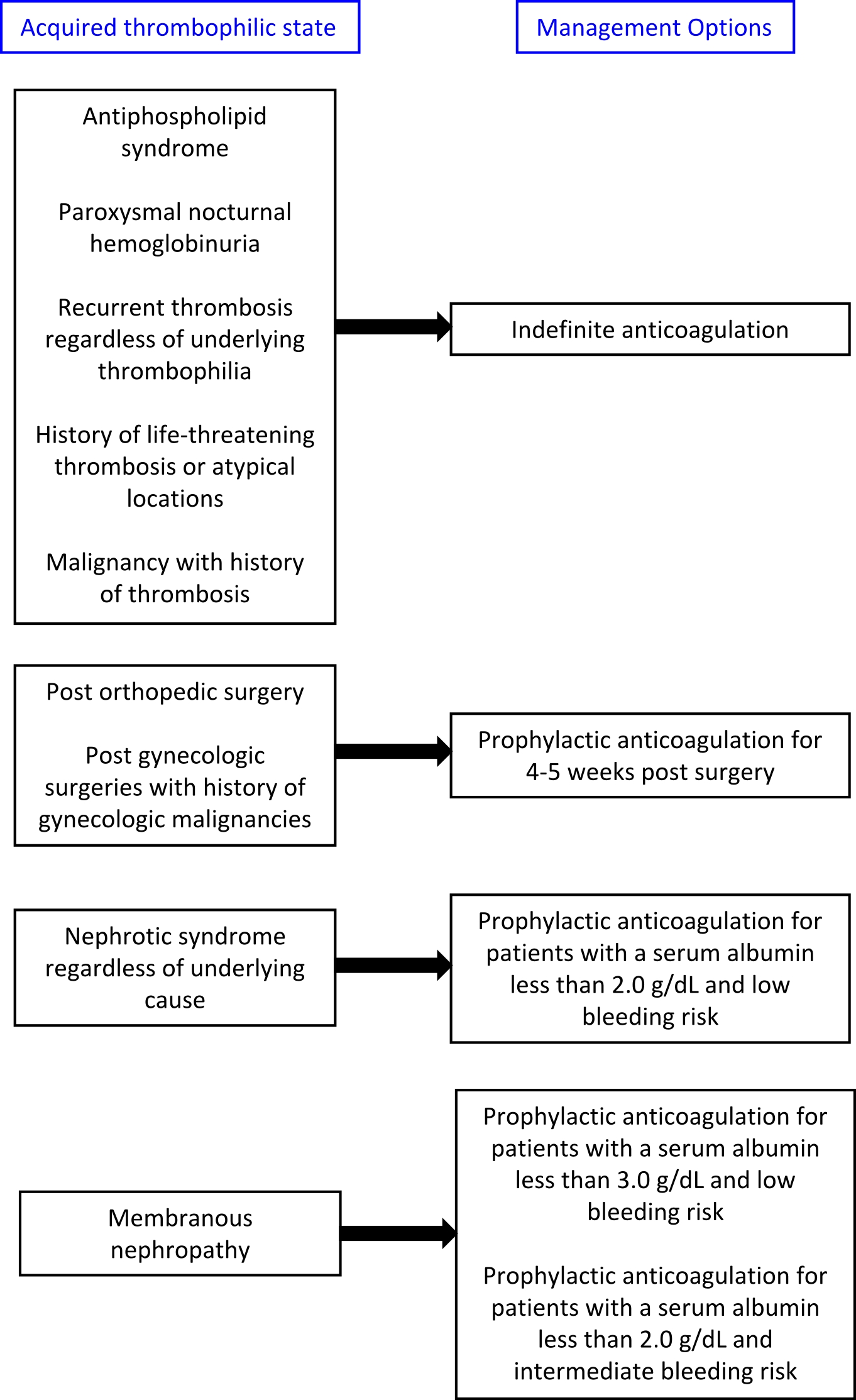
- The American College of Chest Physicians (ACCP) 2016 guidelines:
- It recommends that patients with provoked venous thrombosis from reversible or acquired risk factors should receive 3-6 months of anticoagulation[4]
- Direct oral anticoagulants (DOACs) including direct Xa inhibitors and direct thrombin inhibitors can be used for the long term treatment of most patients with the exceptions of pregnancy, renal insufficiency and malignancy. They may be considered as agents for extended thromboprophylaxis after total hip replacement and total knee replacement. [4] [7] [8]
- Low molecular weight heparin (LMWH): It is recommended for the anticoagulation of the following acquired thrombophilias:
- Malignancy[8]
- Post-surgery prophylaxis[9][10][11]
- The duration of anticoagulation after surgery is variable. General recommendations for thrombophrophylaxis is 7-10 days for standard risk patients and 10-35 days for higher risk patients and for patients undergoing abdominal and pelvic surgeries for gynecologic malginancies [12]
- Pregnancy and postpartum[7]
- Acute thrombosis during pregnancy should be anticoagulated for the remainder of the their pregnancy and 6 weeks postpartum for a minimum of 3 months.
- Warfarin: It is the agent of choice for prophylactic anticoagulation in patients with nephrotic syndrome. [13][14]
- Lee et al created an online tool to assist in evaluating the benefits and risks of prophylactic anticoagulation in patients with membranous nephropathy.
- Response to anticoagulation can be monitored clinically with repeat ultrasongraphy for deep vein thrombosis or measuring D-dimer levels after treatment
- Thromboprophylaxis or indefinate anticoagulation may be required for certain inherited thrombophilias.
Acute Thrombosis
- The initial management of acute VTE, deep vein thrombosis (DVT) or pulmonary embolism (PE) is same in all the patients irrespective of the type of thrombophilias, heritable or acquired thrombophilia.
- Management involves anticoagulation with IV unfractionated heparin (UFH) or low molecular weight heparin with an eventual transition to oral anticoagulation as prompt anticoagulation with heparin or LMWH provides a quick therapeutic range to avoid the progression of thrombosis and reduce associated mortality.
- Treatment phases: Treatment duration following VTE divides into three phases:
- Acute: few days following the event
- Intermediate: short-term anticoagulation for three months
- Chronic: Long-term anticoagulation for more than 3 months
- Treatment regimen:
- An initial anticoagulation with unfractionated or low-molecular-weight heparin for a minimum of 5 days is followed by oral anticoagulation with warfarin or other vitamin K antagonists for 6 months at a target International Normalized Ratio (INR) of 2.5 (range 2.0±3.0).
- Heparin with adjustment for renal impairment is continued until the prothrombin time is in the therapeutic range namely an International Normalized Ratio (INR) of 2.0±3.0.
- Unfractionated heparin: The partial thromboplastin time requires close monitoring and the dose should be adjusted each time based on the value.
- LMWH does not require monitoring though occasionally for patients who are morbidly obese can be monitored by checking anti-Factor Xa levels.
- Warfarin: It is started within the first 24 hours, requires the PT/INR monitoring which should be maintained in therapeutic range (INR typically between 2 and 3).
- Newer Direct Oral Anticoagulants (DOACs): It includes dabigatran, edoxaban, rivaroxaban, and apixaban. Dabigatran is a direct thrombin inhibitor, while the other three are direct factor Xa inhibitors. The American Society of Hematology (ASH) has recommended in 2018 that the direct oral anticoagulants (DOACs) can be used in certain circumstances for the management of acute VTE while taking into consideration the risk of bleeding, renal impairment, and comorbidities that may reduce their efficacy (e.g., morbid obesity). With the DOACs, no routine blood monitoring is necessary; however, medication compliance/adherence remains paramount to assuring the efficacy of these medications.
- If there is any contraindication to anticoagulation or high bleeding risk, the mechanical prophylaxis of lower extremities including intermittent compression devices and graduated elastic compression stockings should be employed.
- Simple distal DVT: The recommended duration of anticoagulation is typically 3 months for an initial provoked thrombosis in the form of the distal DVT.
- Extensive thrombosis: Atleast 3-6 months duration or longer is considered in the first episode of provoked thrombosis with extensive clot burden, massive pulmonary embolism associated with hemodynamic compromise, or in patients with multiple persistent provoking risk factors.
- Unprovoked thrombosis: The duration of therapy is typically longterm and can be continued for lifelong.
- Malignancy: Patients with active malignancy as a provoking risk factor for thrombosis should typically continue anticoagulation therapy for 6 months or longer. Historically, LMWH has been preferred in those with thrombosis and malignancy. However, the newer guidelines are now incorporating the use of certain direct oral anticoagulants (e.g., edoxaban) for management in patients with malignancy.
Antithrombin deficiency
- The action of heparin is to lower the antithrombin levels by approximately 30% over the use of several days which can subsequently makes the antithrombin deficient patients resistant to heparin and require the large doses.
- Antithrombin concentrate: It is used effectively in the following conditions:
- Antithrombin deficiency
- Acute venous thrombosis
- Unusually severe thrombosis
- Difficulty in achieving adequate anticoagulation
- Recurrent thrombosis despite adequate anticoagulation
- Antithrombin-deficient patients with contraindication to the anticoagulation use.
- Dosage regimen:
- The infusion of 50 units/kg body weight of plasma-derived antithrombin concentrate will raise the plasma antithrombin level to approximately 120% in a congenitally deficient individual with a baseline value of 50%.
- One unit is defined as the amount in 1 mL of pooled normal human plasma with a biological half-life approximates 2.8–4.8 days.
- An administration of 60% of the initial dose at 24 h intervals is recommended to maintain antithrombin levels in the normal range; and plasma levels should be monitored to ensure that they remain above 80% .
- Recovery of plasma-derived antithrombin concentrate in vivo in patients with antithrombin deficiency is 1.4%�2.7% unit�1 kg�1.
- However, the low recovery rate is found in patients with acute thrombotic events and those receiving heparin therapy.
Protein C deficiency
- Protein C deficiency in neonates is controllable with protein C replacement from fresh frozen plasma (FFP) or human plasma-derived, viral inactivated protein C concentrate. However, protein C replacement can be expensive leading to the use of anticoagulation therapies in specific settings such as VTEs occurring in children.
- Thereby, anticoagulation treatments with high-intensity warfarin or low-molecular-weight heparin are other considered options.
- Treatment regimen: Oral anticoagulation started under the cover of full heparinization; while the dose of warfarin should be increased gradually, starting from a relatively low level such as 2mg for the first 3 days and then increasing amounts of 2–3mg day�1 until therapeutic anticoagulation is achieved.
- However, protein C administration either in the form of fresh frozen plasma or protein C concentrate can provide protection against recurrent skin necrosis until a stable level of anticoagulation is achieved.
Pregnancy
- In general, pregnant women with anticoagulant factor deficiencies and a personal or familial history of thrombosis should be considered for anticoagulant prophylaxis. Pregnant women with antithrombin deficiency appear to have an unusually high risk for thromboembolism, and should receive anticoagulant prophylaxis throughout pregnancy. Antithrombin concentrates are available but should be reserved for use during labor, delivery or obstetric complications where the risks of bleeding from anticoagulation are unacceptable. During pregnancy, adjusted-dose unfractionated heparin or low-molecular-weight heparin administered by the subcutaneous route has been the anticoagulant of choice because its efficacy and safety for the fetus are established. Heparin can produce bone loss but is not associated with the embryopathy that can result from the early administration of warfarin. LMWH is an attractive alternative to unfractionated heparin in this setting because of its better bioavailability, longer half-life, and ease of administration. Enoxaparin, for example, is rated by the US Food and Drug Administration (FDA) as pregnancy category B; while not FDA-approved for use in pregnancy, it appears to be safe and effective. The dose and duration of LMWH therapy in pregnancy however are uncertain since appropriately designed clinical trials have not yet been performed. The following approach is suggested.
- Patients considered to be at high thrombotic risk should receive full-dose heparin or LMWH by subcutaneous injection every 12 h for the duration of pregnancy and approximately 6 weeks postpartum. For unfractionated heparin, the dose should be adjusted to maintain the 6 h postinjection activated partial thromboplastin time (aPTT) at 1.5 times the control value. For LMWH, firm guidelines regarding the need for monitoring have not been established. In view of the increase in total body plasma volume during pregnancy, intermittent monitoring of plasma heparin levels by anti- FXa assay should be performed, starting in the second trimester. The goal is a plasma heparin concentration of 0.5 1.0UmL�1 2–3 h after injection.
- Women with a personal or family history of thrombosis and considered to be at intermediate risk can be treated with lower subcutaneous doses of heparin: 5000–10 000 units of unfractionated heparin subcutaneously every 12 h; or prophylactic doses of LMWH every 12 h. Therapy should be started during the second or third trimester and continued for approximately 6 weeks into the postpartum period.
- Low-risk patients (e.g. asymptomatic carriers without a family history of recurrent thromboses) should be observed closely throughout the pregnancy.
Chronic venous ulceration (CVUs)
- Lymphatic destruction, excessive colloid filtration resulting in regional edema, fibrin deposition, a diffusion barrier to oxygen, and infiltration of lymphocytes and neutrophils occurs in the setting of elevated lower extremity venous pressure, venous hypertension and chronic venous reflux which further promote inflammation and skin changes of the lower extremity that predispose the skin to ulceration.
- Compression therapy is the mainstay of treatment for CVUs with up to 96% of ulcers healing.
- The current CHEST guidelines based on randomized control trials show a significant benefit to the use of 30 mmhg-40 mmhg compression stockings for at least 2 years in decreasing progression of venous disease after an episode of DVT.
- Surgical correction of venous reflux does not seem to aid in ulcer healing of primary CVUs; however, it does seem to reduce ulcer recurrence rates.
- Miscellaneous: Agents such as pentoxifylline and rutosides has been identified by the CHEST guidelines to show some benefit in the healing of venous ulcers.
Duration of Anticoagulant Therapy
- After a first venous thromboembolism, anticoagulant therapy is generally administered for 6 months. A shorter period of treatment may be acceptable when the thrombus is confined to distal veins (calf veins) and if there is evidence of a temporary risk factor that is no longer present. It is recommended that when there is a persisting thrombotic risk factor such as cancer or already identified high-risk thrombophilic defects (e.g. type I or type II reactive site antithrombin deficiency or combined defects), consideration should be given to extending the usual period of anticoagulation on an individual patient basis (Grade B recommendation).
- Identification of the most prevalent forms of heritable thrombophilia, heterozygosity for factor V Leiden or prothrombin G20210A, should not influence decisions about the duration of anticoagulant therapy (Grade B recommendation).
- Discontinuing therapy, before established guidelines on duration, can increase the risk of recurrent thrombosis. Medication compliance/adherence is very important to decrease the risk of recurrence. In patients with recurrent DVT or PE, the duration of therapy becomes more complex, and the determination of strong provoking or transient risk factors is important for guiding treatment.
- Anticoagulation with thrombosis in pregnancy, perioperatively in those with thrombosis (especially orthopedic surgery), thrombosis in those with hereditary or acquired thrombophilia, and management of patients with recurrent or "breakthrough" thrombosis (despite therapeutic anticoagulation) becomes significantly more complicated. These cases are best managed under the guidance of a hematologist consultant.
Management of recurrent venous thrombosis
- Different anticoagulants and antiplatelets are available to prevent recurrent VTE. They include vitamin K antagonist (VKA), aspirin (as assessed in the WARFASA and ASPIRE trials), rivaroxaban (EINSTEIN trial), dabigatran (RE-MEDY and RE-SONATE trials), and apixaban (AMPLIFY trial). Additional considerations are prudent regarding special populations. The CLOT trial assessed low molecular weight heparin against warfarin in cancer patients. Heparin did not show teratogenicity and is FDA-approved during pregnancy and the postpartum period. Prevention of thrombotic events includes compression stockings and mobility. Rosuvastatin prevents the occurrence of VTE.
- When recurrent events have occurred while the patient was no longer anticoagulated, it is sufficient to reintroduce coumarin at a target INR of 2´5 after initial treatment with heparin, but when a recurrent event has occurred while the patient was on anticoagulants and their INR was within the target range of 2´0±3´0, an increase in the intensity of anticoagulation to a target INR of 3´5 (range 3´0±4´0) is indicated (Grade C recommendation).
- In general, patients who have had two or more apparently spontaneous venous thrombotic events require consideration for indefinite anticoagulant thromboprophylaxis (Grade C recommendation).
- Risk stratification tools for the estimation of VTE recurrence in cancer include the COMPASS-CAT, Ottawa (Louzada) and Khorana scores.
- However, patients who have had recurrent thrombotic events in association with identifiable prothrombotic triggers (for example pregnancy, surgery, oestrogen use) and in whom those prothrombotic triggers are no longer present may not require indefinite anticoagulant thromboprophylaxis but do require prophylaxis during high-risk situations.
- Factors such as male gender, age, proximal compared to distal deep vein thrombosis which has a higher thrombotic burden, increased d-dimer, and unprovoked VTE implicate a higher recurrence rate and can trigger extended coagulation.
References
- ↑ DeLoughery TG. Hemostasis and Thrombosis: Springer International Publishing; 2014.
- ↑ Cohoon KP, Heit JA (2014). "Inherited and secondary thrombophilia". Circulation. 129 (2): 254–7. doi:10.1161/CIRCULATIONAHA.113.001943. PMC 3979345. PMID 24421360.
- ↑ Seligsohn U, Lubetsky A (2001). "Genetic susceptibility to venous thrombosis". N Engl J Med. 344 (16): 1222–31. doi:10.1056/NEJM200104193441607. PMID 11309638.
- ↑ 4.0 4.1 4.2 Streiff MB, Agnelli G, Connors JM, Crowther M, Eichinger S, Lopes R; et al. (2016). "Guidance for the treatment of deep vein thrombosis and pulmonary embolism". J Thromb Thrombolysis. 41 (1): 32–67. doi:10.1007/s11239-015-1317-0. PMC 4715858. PMID 26780738.
- ↑ Martinelli I, Franchini M, Mannucci PM (2008). "How I treat rare venous thromboses". Blood. 112 (13): 4818–23. doi:10.1182/blood-2008-07-165969. PMID 18805965.
- ↑ De Stefano V, Grandone E, Martinelli I (2013). "Recommendations for prophylaxis of pregnancy-related venous thromboembolism in carriers of inherited thrombophilia. Comment on the 2012 ACCP guidelines". J Thromb Haemost. 11 (9): 1779–81. doi:10.1111/jth.12330. PMID 23789890.
- ↑ 7.0 7.1 Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; et al. (2012). "VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e691S–736S. doi:10.1378/chest.11-2300. PMC 3278054. PMID 22315276.
- ↑ 8.0 8.1 Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M; et al. (2003). "Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer". N Engl J Med. 349 (2): 146–53. doi:10.1056/NEJMoa025313. PMID 12853587. Review in: ACP J Club. 2004 Jan-Feb;140(1):10 Review in: J Fam Pract. 2003 Nov;52(11):843-4
- ↑ Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S; et al. (2012). "Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e278S–325S. doi:10.1378/chest.11-2404. PMC 3278063. PMID 22315265.
- ↑ Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A; et al. (2002). "Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer". N Engl J Med. 346 (13): 975–80. doi:10.1056/NEJMoa012385. PMID 11919306.
- ↑ Agnelli G (2004). "Prevention of venous thromboembolism in surgical patients". Circulation. 110 (24 Suppl 1): IV4–12. doi:10.1161/01.CIR.0000150639.98514.6c. PMID 15598646.
- ↑ Muntz J (2010). "Duration of deep vein thrombosis prophylaxis in the surgical patient and its relation to quality issues". Am J Surg. 200 (3): 413–21. doi:10.1016/j.amjsurg.2009.05.045. PMID 20409525.
- ↑ Glassock RJ (2007). "Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum". J Am Soc Nephrol. 18 (8): 2221–5. doi:10.1681/ASN.2006111300. PMID 17599972.
- ↑ Lee T, Biddle AK, Lionaki S, Derebail VK, Barbour SJ, Tannous S; et al. (2014). "Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy". Kidney Int. 85 (6): 1412–20. doi:10.1038/ki.2013.476. PMC 4040154. PMID 24336031.