Tezacaftor / ivacaftor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Tezacaftor / ivacaftor is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tezacaftor / ivacaftor in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Tezacaftor / ivacaftor and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Tezacaftor / ivacaftor
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
There is limited information regarding Tezacaftor / ivacaftor Clinical Studies in the drug label.
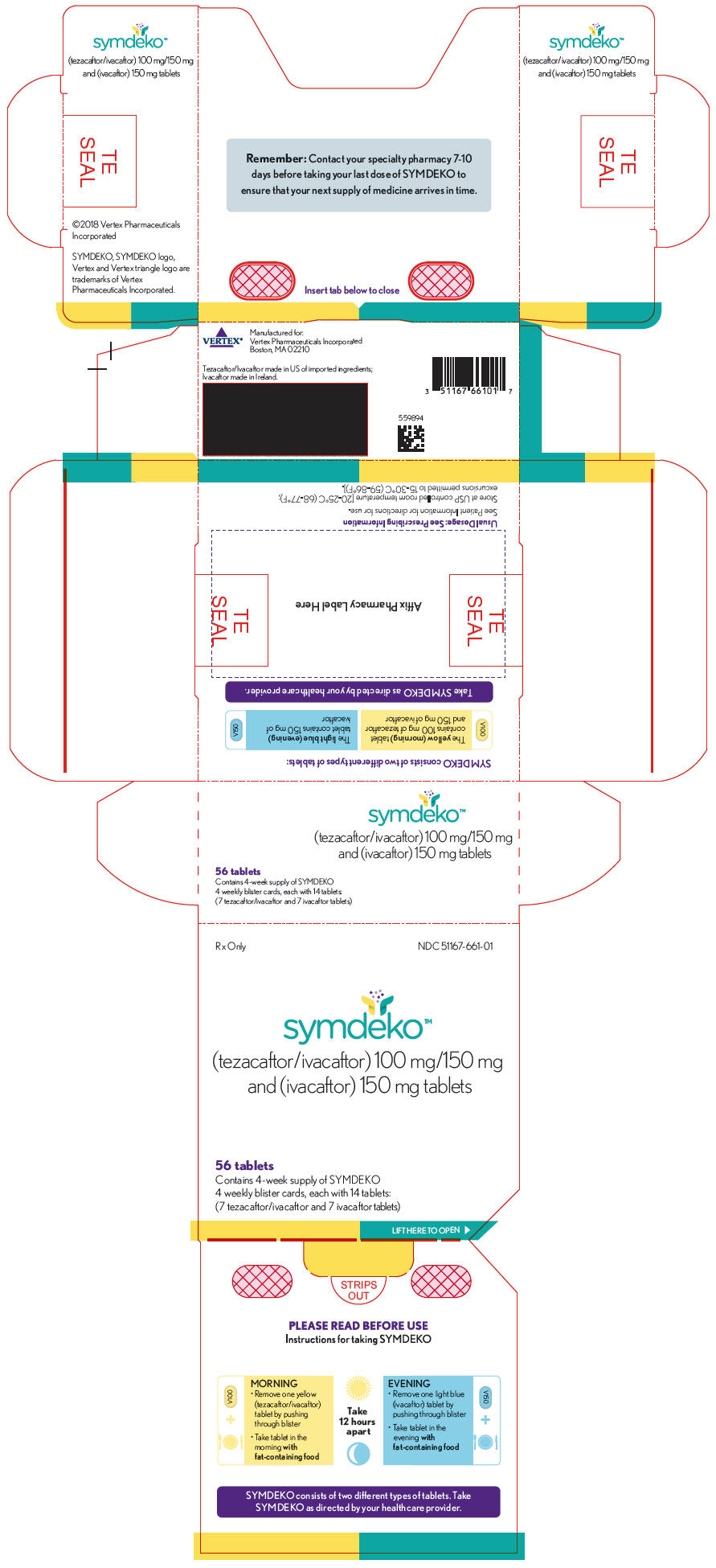
How Supplied
- SYMDEKO is co-packaged as a tezacaftor/ivacaftor fixed-dose combination tablet and an ivacaftor tablet. The tezacaftor/ivacaftor fixed dose combination tablets are supplied as yellow, capsule-shaped tablets containing 100 mg of tezacaftor and 150 mg of ivacaftor. Each tablet is debossed with "V100" on one side and plain on the other. Ivacaftor tablets are supplied as light blue, film-coated, capsule-shaped tablets containing 150 mg of ivacaftor. Each tablet is printed with the characters "V150" on one side and plain on the other. SYMDEKO is supplied as:
- 56-count tablet carton containing a 4-week supply (4 weekly wallets, each with 14 tablets)
- NDC 51167-661-01
Storage
- Store at 20-25ºC (68-77ºF); excursions permitted to 15-30ºC (59-86ºF).
Images
Drug Images
{{#ask: Page Name::Tezacaftor / ivacaftor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tezacaftor / ivacaftor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
Transaminase (ALT or AST) Elevations and Monitoring
- Inform patients that elevation in liver tests has occurred in patients treated with SYMDEKO or with ivacaftor alone. Transaminases (ALT and AST) should be assessed prior to initiating SYMDEKO, every 3 months during the first year of treatment, and annually thereafter. More frequent monitoring should be considered in patients with a history of transaminase elevations.
Drug Interactions with CYP3A Inducers and Inhibitors
- Ask patients to tell you all the medications they are taking including any herbal supplements or vitamins. Co-administration of SYMDEKO with strong CYP3A inducers (e.g., rifampin, St. John's wort) is not recommended, as they may reduce the therapeutic effectiveness of SYMDEKO. Adjustment of the dose to one tablet of tezacaftor 100 mg/ivacaftor 150 mg twice a week, taken approximately 3 to 4 days apart is recommended when co-administered with strong CYP3A inhibitors, such as ketoconazole. Advise the patient not to take the evening dose of ivacaftor 150 mg. Dose reduction to one tablet of tezacaftor 100 mg/ivacaftor 150 mg or ivacaftor 150 mg, taken on alternate days in the morning is recommended when co-administered with moderate CYP3A inhibitors, such as fluconazole. Advise the patient not to take the evening dose of ivacaftor 150 mg. Food or drink containing grapefruit or Seville oranges should be avoided.
Cataracts
- Inform patients that abnormality of the eye lens (cataract) has been noted in some children and adolescents receiving SYMDEKO or with ivacaftor alone. Baseline and follow-up ophthalmological examinations should be performed in pediatric patients initiating treatment with SYMDEKO.
Use in Patients with Hepatic Impairment
- Inquire and/or assess whether patients have liver impairment. Adjust the dose in patients with moderately impaired hepatic function (Child-Pugh Class B, score 7-9) to one tablet of tezacaftor 100 mg/ivacaftor 150 mg once daily in the morning and advise the patient not to take the evening dose of ivacaftor 150 mg. SYMDEKO has not been studied in patients with severe hepatic impairment (Child-Pugh Class C, score 10-15); however, exposure is expected to be substantially higher than that observed in patients with moderate hepatic impairment. When benefits are expected to outweigh the risks, SYMDEKO should be used with caution in patients with severe hepatic impairment at a dose of one tablet of tezacaftor 100 mg/ivacaftor 150 mg once daily in the morning or less frequently. Advise the patient not to take the evening dose of ivacaftor 150 mg. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh Class A, score 5-6).
Administration
- Inform patients that SYMDEKO is best absorbed by the body when taken with food that contains fat. A typical CF diet will satisfy this requirement. Examples include eggs, butter, peanut butter, cheese pizza, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc..
- Patients should be informed about what to do in the event they miss a dose of SYMDEKO or ivacaftor:
- If 6 hours or less have passed since the time SYMDEKO is usually taken, patients should be instructed to take the prescribed dose of SYMDEKO with fat-containing food as soon as possible.
- If more than 6 hours have passed since the time SYMDEKO is usually taken, the missed dose should NOT be taken and the patient should resume the usual dosing schedule.
- Patients should be advised to contact their health care provider if they have questions.

Precautions with Alcohol
Alcohol-Tezacaftor / ivacaftor interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Symdeko
Look-Alike Drug Names
There is limited information regarding Tezacaftor / ivacaftor Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.