Azelastine (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Azelastine (ophthalmic) is a {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
For the treatment of itching of the eye associated with allergic conjunctivitis.
Dosage
- The recommended dose is one drop instilled into each affected eye twice a day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Azelastine (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Azelastine (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Azelastine (ophthalmic) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Azelastine (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Azelastine (ophthalmic) in pediatric patients.
Contraindications
- Azelastine hydrochloride ophthalmic solution is contraindicated in persons with known or suspected hypersensitivity to any of its components.
Warnings
- Azelastine hydrochloride ophthalmic solution is for ocular use only and not for injection or oral use.
Adverse Reactions
Clinical Trials Experience
- In controlled multiple-dose studies where patients were treated for up to 56 days, the most frequently reported adverse reactions were transient eye burning/stinging (approximately 30%), headaches (approximately 15%) and bitter taste (approximately 10%). The occurrence of these events was generally mild.
- The following events were reported in 1 - 10% of patients: asthma, conjunctivitis, dyspnea, eye pain, fatigue, influenza-like symptoms, pharyngitis, pruritus, rhinitis and temporary blurring. Some of these events were similar to the underlying disease being studied.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Azelastine (ophthalmic) in the drug label.
Drug Interactions
There is limited information regarding Azelastine (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects: Pregnancy Category C.
- Azelastine hydrochloride has been shown to be embryotoxic, fetotoxic, and teratogenic (external and skeletal abnormalities) in mice at an oral dose of 68.6 mg/kg/day (57,000 times the recommended ocular human use level). At an oral dose of 30 mg/kg/day (25,000 times the recommended ocular human use level), delayed ossification (undeveloped metacarpus), and the incidence of 14th rib were increased in rats. At 68.6 mg/kg/day (57,000 times the maximum recommended ocular human use level) azelastine hydrochloride caused resorption and fetotoxic effects in rats. The relevance to humans of these skeletal findings noted at only high drug exposure levels in unknown.
- There are no adequate and well-controlled studies in pregnant women. Azelastine hydrochloride ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Azelastine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Azelastine (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether azelastine hydrochloride is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when azelastine hydrochloride ophthalmic solution is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 3 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger adult patients.
Gender
There is no FDA guidance on the use of Azelastine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Azelastine (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Azelastine (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Azelastine (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Azelastine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Azelastine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Azelastine (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Azelastine (ophthalmic) in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Azelastine (ophthalmic) in the drug label.
Pharmacology
| |
Azelastine (ophthalmic)
| |
| Systematic (IUPAC) name | |
| (RS)-4-[(4-chlorophenyl)methyl]-2- (1-methylazepan-4-yl)-phthalazin-1-one | |
| Identifiers | |
| CAS number | |
| ATC code | R01 R06AX19 (WHO), S01GX07 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 381.898 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 40% (intranasal) |
| Metabolism | ? |
| Half life | 22 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
Rx Only (US) |
| Routes | intranasal, ocular |
Mechanism of Action
There is limited information regarding Azelastine (ophthalmic) Mechanism of Action in the drug label.
Structure
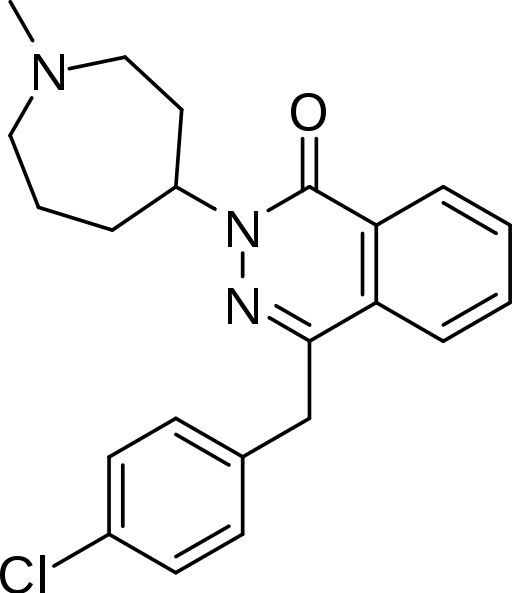
Azelastine Hydrochloride Ophthalmic Solution, 0.05% is a sterile ophthalmic solution containing azelastine hydrochloride, a relatively selective H1-receptor antagonist for topical administration to the eyes. Azelastine hydrochloride is a white crystalline powder with a molecular weight of 418.37. Azelastine hydrochloride is sparingly soluble in water, methanol and propylene glycol, and slightly soluble in ethanol, octanol, and glycerine. Azelastine hydrochloride is a racemic mixture with a melting point of 225°C. The chemical name for azelastine hydrochloride is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride and is represented by the following chemical structure:

- Each mL of azelastine hydrochloride ophthalmic solution contains: Active: 0.5 mg azelastine hydrochloride, equivalent to 0.457 mg of azelastine base; Preservative: 0.125 mg benzalkonium chloride; Inactives: disodium edetate dihydrate, hydroxypropylmethylcellulose, sodium hydroxide, sorbitol solution and water for injection. It has a pH of approximately 5.0 to 6.5 and an osmolality of approximately 271 to 312 mOsmol/L.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Azelastine (ophthalmic) in the drug label.
Pharmacokinetics
- Absorption of azelastine following ocular administration was relatively low. A study in symptomatic patients receiving one drop of azelastine hydrochloride ophthalmic solution in each eye two to four times a day (0.06 to 0.12 mg azelastine hydrochloride) demonstrated plasma concentrations of azelastine hydrochloride to generally be between 0.02 and 0.25 ng/mL after 56 days of treatment. Three of nineteen patients had quantifiable amounts of N-desmethylazelastine that ranged from 0.25 - 0.87 ng/mL at Day 56.
- Based on intravenous and oral administration, the elimination half-life, steady-state volume of distribution and plasma clearance were 22 hours, 14.5 L/kg and 0.5 L/h/kg, respectively. Approximately 75% of an oral dose of radiolabeled azelastine hydrochloride was excreted in the feces with less than 10% as unchanged azelastine. Azelastine hydrochloride is oxidatively metabolized to the principal metabolite, N-desmethylazelastine, by the cytochrome P450 enzyme system. In vitro studies in human plasma indicate that the plasma protein binding of azelastine and N-desmethylazelastine are approximately 88% and 97% respectively.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Azelastine hydrochloride administered orally for 24 months was not carcinogenic in rats and mice at doses up to 30 mg/kg/day and 25 mg/kg/day, respectively. Based on a 30 µL drop size, these doses were approximately 25,000 and 21,000 times higher than the maximum recommended ocular human use level of 0.001 mg/kg/day for a 50 kg adult.
- Azelastine hydrochloride showed no genotoxic effects in the Ames test, DNA repair test, mouse lymphoma forward mutation assay, mouse micronucleus test, or chromosomal aberration test in rat bone marrow. Reproduction and fertility studies in rats showed no effects on male or female fertility at oral doses of up to 25,000 times the maximum recommended ocular human use level. At 68.6 mg/kg/day (57,000 times the maximum recommended ocular human use level), the duration of the estrous cycle was prolonged and copulatory activity and the number of pregnancies were decreased. The numbers of corpora lutea and implantations were decreased; however, the implantation ratio was not affected.
Clinical Studies
- In a conjunctival antigen challenge study, azelastine hydrochloride ophthalmic solution was more effective than its vehicle in preventing itching associated with allergic conjunctivitis. Azelastine hydrochloride ophthalmic solution had a rapid (within 3 minutes) onset of effect and a duration of effect of approximately 8 hours for the prevention of itching.
In environmental studies, adult and pediatric, patients with seasonal allergic conjunctivitis were treated with azelastine hydrochloride ophthalmic solution for two to eight weeks. In these studies, azelastine hydrochloride ophthalmic solution was more effective than its vehicle in relieving itching associated with allergic conjunctivitis.
How Supplied
- Azelastine Hydrochloride Ophthalmic Solution, 0.05% is supplied as follows: 6 mL (NDC# 60505-0578-4) solution in a translucent 11 mL HDPE container with a LDPE dropper tip, and a white HDPE screw cap.
Storage
- Store UPRIGHT at 20º-25ºC (68º-77ºF).
Images
Drug Images
{{#ask: Page Name::Azelastine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Azelastine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- To prevent contaminating the dropper tip and solution, care should be taken not to touch any surface, the eyelids, or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use. This product is sterile when packaged.
- Patients should be advised not to wear a contact lens if their eye is red. Azelastine hydrochloride ophthalmic solution should not be used to treat contact lens related irritation. The preservative in azelastine hydrochloride ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red, should be instructed to wait at least ten minutes after instilling azelastine hydrochloride ophthalmic solution before they insert their contact lenses.
Precautions with Alcohol
- Alcohol-Azelastine (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- AZELASTINE HYDROCHLORIDE®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "azelastine hydrochloride solution/ drops".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Azelastine (ophthalmic)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Azelastine (ophthalmic) |Label Name=Azelastine (ophthalmic)11.png
}}
{{#subobject:
|Label Page=Azelastine (ophthalmic) |Label Name=Azelastine (ophthalmic)11.png
}}