Sitagliptin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
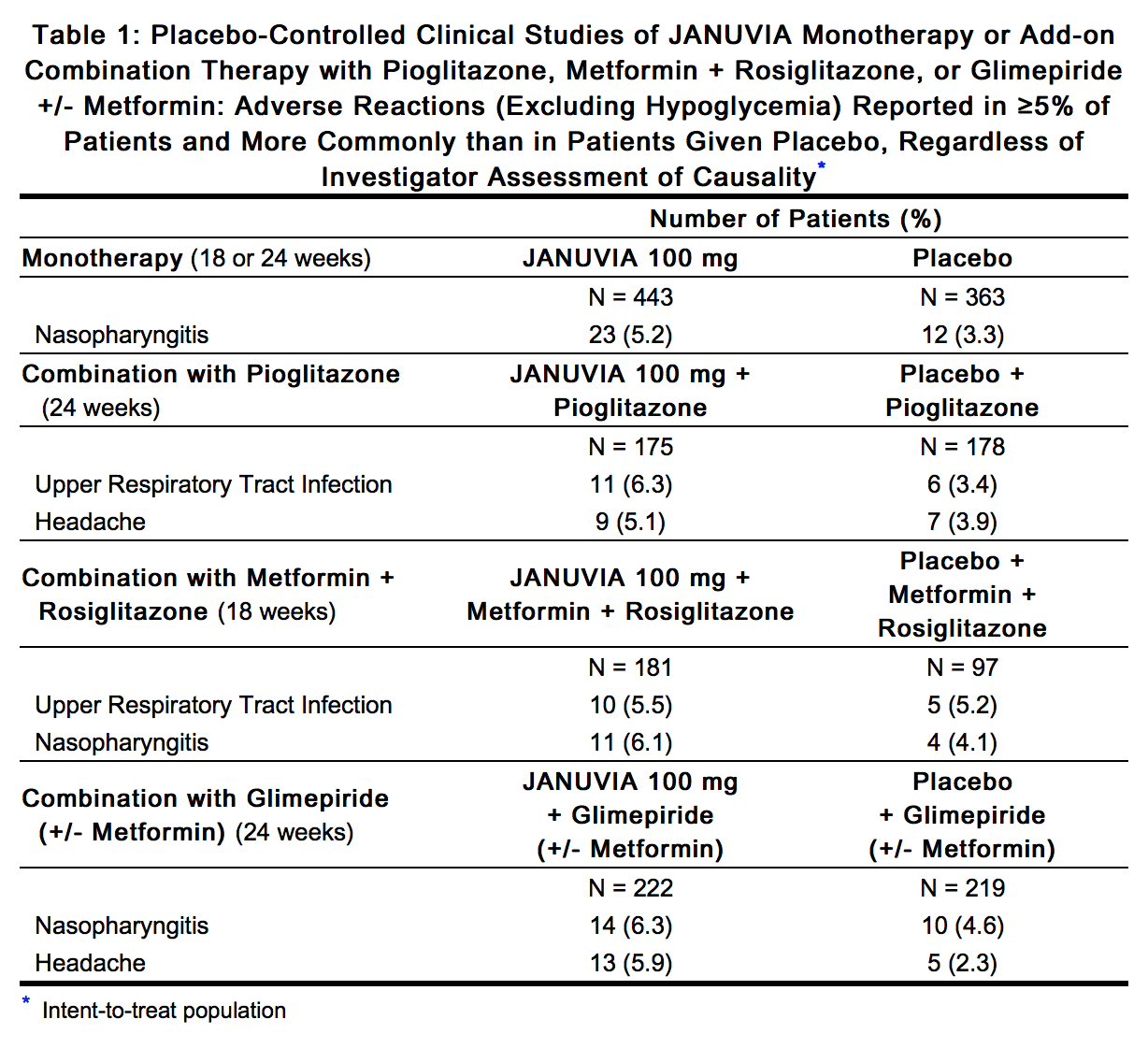
Sitagliptin is a dipeptidyl peptidase-4 inhibitor that is FDA approved for the {{{indicationType}}} of type 2 diabetes mellitus. Common adverse reactions include hypoglycemia, headache, nasopharyngitis, and upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Monotherapy and Combination Therapy for Type 2 Diabetes Mellitus
- Dosing Information
- The recommended dose of JANUVIA is 100 mg once daily. JANUVIA can be taken with or without food.
- Patients with Renal Insufficiency
- For patients with mild renal insufficiency (creatinine clearance [CrCl] greater than or equal to 50 mL/min, approximately corresponding to serum creatinine levels of less than or equal to 1.7 mg/dL in men and less than or equal to 1.5 mg/dL in women), no dosage adjustment for JANUVIA is required.
- For patients with moderate renal insufficiency (CrCl greater than or equal to 30 to less than 50 mL/min, approximately corresponding to serum creatinine levels of greater than 1.7 to less than or equal to 3.0 mg/dL in men and greater than 1.5 to less than or equal to 2.5 mg/dL in women), the dose of JANUVIA is 50 mg once daily.
- For patients with severe renal insufficiency (CrCl less than 30 mL/min, approximately corresponding to serum creatinine levels of greater than 3.0 mg/dL in men and greater than 2.5 mg/dL in women) or with end-stage renal disease (ESRD) requiring hemodialysis or peritoneal dialysis, the dose of JANUVIA is 25 mg once daily. JANUVIA may be administered without regard to the timing of dialysis.
- Because there is a need for dosage adjustment based upon renal function, assessment of renal function is recommended prior to initiation of JANUVIA and periodically thereafter. Creatinine clearance can be estimated from serum creatinine using the Cockcroft-Gault formula. There have been postmarketing reports of worsening renal function in patients with renal insufficiency, some of whom were cascribed inappropriate doses of sitagliptin.
- Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
- When JANUVIA is used in combination with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sitagliptin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sitagliptin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of JANUVIA in pediatric patients under 18 years of age have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sitagliptin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sitagliptin in pediatric patients.
Contraindications
- History of a serious hypersensitivity reaction to sitagliptin, such as anaphylaxis or angioedema.
Warnings
- Pancreatitis
- There have been postmarketing reports of acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, in patients taking JANUVIA. After initiation of JANUVIA, patients should be observed carefully for signs and symptoms of pancreatitis. If pancreatitis is suspected, JANUVIA should promptly be discontinued and appropriate management should be initiated. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JANUVIA.
- Renal Impairment
- Assessment of renal function is recommended prior to initiating JANUVIA and periodically thereafter. A dosage adjustment is recommended in patients with moderate or severe renal insufficiency and in patients with ESRD requiring hemodialysis or peritoneal dialysis. Caution should be used to ensure that the correct dose of JANUVIA is prescribed for patients with moderate (creatinine clearance ≥30 to <50 mL/min) or severe (creatinine clearance <30 mL/min) renal impairment.
- There have been postmarketing reports of worsening renal function, including acute renal failure, sometimes requiring dialysis. A subset of these reports involved patients with renal insufficiency, some of whom were prescribed inappropriate doses of sitagliptin. A return to baseline levels of renal insufficiency has been observed with supportive treatment and discontinuation of potentially causative agents. Consideration can be given to cautiously reinitiating JANUVIA if another etiology is deemed likely to have precipitated the acute worsening of renal function.
- JANUVIA has not been found to be nephrotoxic in preclinical studies at clinically relevant doses, or in clinical trials.
- Use with Medications Known to Cause Hypoglycemia
- When JANUVIA was used in combination with a sulfonylurea or with insulin, medications known to cause hypoglycemia, the incidence of hypoglycemia was increased over that of placebo used in combination with a sulfonylurea or with insulin. Therefore, a lower dose of sulfonylurea or insulin may be required to reduce the risk of hypoglycemia.
- Hypersensitivity Reactions
- There have been postmarketing reports of serious hypersensitivity reactions in patients treated with JANUVIA. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Onset of these reactions occurred within the first 3 months after initiation of treatment with JANUVIA, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, discontinue JANUVIA, assess for other potential causes for the event, and institute alternative treatment for diabetes.
- Angioedema has also been reported with other dipeptidyl peptidase-4 (DPP-4) inhibitors. Use caution in a patient with a history of angioedema with another DPP-4 inhibitor because it is unknown whether such patients will be predisposed to angioedema with JANUVIA.
- Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with JANUVIA or any other anti-diabetic drug.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Sitagliptin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Sitagliptin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sitagliptin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sitagliptin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sitagliptin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Sitagliptin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Sitagliptin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Sitagliptin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sitagliptin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sitagliptin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sitagliptin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sitagliptin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sitagliptin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Sitagliptin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Sitagliptin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Sitagliptin in the drug label.
Pharmacology
There is limited information regarding Sitagliptin Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Sitagliptin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Sitagliptin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Sitagliptin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Sitagliptin in the drug label.
How Supplied
Storage
There is limited information regarding Sitagliptin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sitagliptin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sitagliptin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Sitagliptin in the drug label.
Precautions with Alcohol
- Alcohol-Sitagliptin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Januvia®[1]
Look-Alike Drug Names
- Januvia® — Enjuvia®[2]
- Januvia® — Jantoven®[2]
- Januvia® — Janumet®[2]
- sitaGLIPtin® — SUMAtriptan®[2]
- sitaGLIPtin® — ZOLMitriptan®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "JANUVIA (sitagliptin) tablet, film coated".
- ↑ 2.0 2.1 2.2 2.3 2.4 "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Sitagliptin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Sitagliptin |Label Name=Sitagliptin11.png
}}
{{#subobject:
|Label Page=Sitagliptin |Label Name=Sitagliptin11.png
}}