Isoflurane: Difference between revisions
No edit summary |
No edit summary |
||
| Line 138: | Line 138: | ||
|fdaPatientInfo=Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration. | |fdaPatientInfo=Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration. | ||
|alcohol=Alcohol-Isoflurane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Isoflurane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | |||
{{LabelImage | |||
|fileName=Isoflurane label.png | |||
}} | }} | ||
Revision as of 03:55, 19 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Isoflurane is a general anesthetic that is FDA approved for the {{{indicationType}}} of general anesthesia. Common adverse reactions include gastrointestinal: nausea, vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- General anesthesia: induction, 1.5 to 3% isoflurane with oxygen or oxygen-nitrous oxide mixture.
- General anesthesia: maintenance, 1 to 2.5% with nitrous oxide, additional 0.5 to 1% with oxygen alone.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Isoflurane in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Isoflurane in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety in children under 2 yr of age has not been established
- General anesthesia: dosage must be individualized
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Isoflurane in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Isoflurane in pediatric patients.
Contraindications
- Known sensitivity to FORANE (isoflurane, USP) or to other halogenated agents. Known or suspected genetic susceptibility to malignant hyperthermia.
Warnings
Perioperative Hyperkalemia
Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatinine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended, as is subsequent evaluation for latent neuromuscular disease.
Malignant Hyperthermia
In susceptible individuals, isoflurane anesthesia may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. The syndrome includes nonspecific features such as muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and unstable blood pressure. (It should also be noted that many of these nonspecific signs may appear with light anesthesia, acute hypoxia, etc.) An increase in overall metabolism may be reflected in an elevated temperature, (which may rise rapidly early or late in the case, but usually is not the first sign of augmented metabolism) and an increased usage of the CO2 absorption system (hot canister). PaO2 and pH may decrease, and hyperkalemia and a base deficit may appear. Treatment includes discontinuance of triggering agents (e.g., isoflurane), administration of intravenous dantrolene sodium, and application of supportive therapy. Such therapy includes vigorous efforts to restore body temperature to normal, respiratory and circulatory support as indicated, and management of electrolyte-fluid-acid-base derangements. (Consult prescribing information for dantrolene sodium intravenous for additional information on patient management). Renal failure may appear later, and urine flow should be sustained if possible.
Since levels of anesthesia may be altered easily and rapidly, only vaporizers producing predictable concentrations should be used. Hypotension and respiratory depression increase as anesthesia is deepened.
Increased blood loss comparable to that seen with halothane has been observed in patients undergoing abortions.
FORANE (isoflurane, USP) markedly increases cerebral blood flow at deeper levels of anesthesia. There may be a transient rise in cerebral spinal fluid pressure, which is fully reversible with hyperventilation.
Adverse Reactions
Clinical Trials Experience
Adverse reactions encountered in the administration of FORANE (isoflurane, USP) are in general dose dependent extensions of pharmacophysiologic effects and include respiratory depression, hypotension and arrhythmias.
Shivering, nausea, vomiting and ileus have been observed in the postoperative period.
As with all other general anesthetics, transient elevations in white blood count have been observed even in the absence of surgical stress. See WARNINGS for information regarding malignant hyperthermia and elevated carboxyhemoglobin levels.
During marketing, there have been rare reports of mild, moderate and severe (some fatal) postoperative hepatic dysfunction and hepatitis.
FORANE (isoflurane, USP) has also been associated with perioperative hyperkalemia (see WARNINGS).
Postmarketing Experience
The following adverse events have been identified during post-approval use of FORANE (isoflurane, USP). Due to the spontaneous nature of these reports, the actual incidence and relationship of FORANE (isoflurane, USP) to these events cannot be established with certainty.
Cardiac Disorders: Cardiac arrest
Hepatobiliary Disorders: Hepatic necrosis, Hepatic failure
Drug Interactions
There is limited information regarding Isoflurane Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Isoflurane has been shown to have a possible anesthetic-related fetotoxic effect in mice when given in doses 6 times the human dose. There are no adequate and well-controlled studies in pregnant women. Isoflurane should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Isoflurane in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Isoflurane during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Isoflurane in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Isoflurane in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Isoflurane in geriatric settings.
Gender
There is no FDA guidance on the use of Isoflurane with respect to specific gender populations.
Race
There is no FDA guidance on the use of Isoflurane with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Isoflurane in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Isoflurane in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Isoflurane in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Isoflurane in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Isoflurane Administration in the drug label.
Monitoring
There is limited information regarding Isoflurane Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Isoflurane and IV administrations.
Overdosage
In the event of overdosage, or what may appear to be overdosage, the following action should be taken:
Stop drug administration, establish a clear airway, and initiate assisted or controlled ventilation with pure oxygen.
Pharmacology
| |
| |
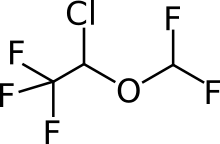
Isoflurane
| |
| Systematic (IUPAC) name | |
| (RS)-2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane OR (RS)-1-chloro-2,2,2-trifluoroethyl difluoromethyl ether | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 184.5 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Isoflurane Mechanism of Action in the drug label.
Structure
FORANE (isoflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2, 2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:

Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Isoflurane has a mildly pungent, musty, ethereal odor. Samples stored in indirect sunlight in clear, colorless glass for five years, as well as samples directly exposed for 30 hours to a 2 amp, 115 volt, 60 cycle long wave U.V. light were unchanged in composition as determined by gas chromatography. Isoflurane in one normal sodium methoxide-methanol solution, a strong base, for over six months consumed essentially no alkali, indicative of strong base stability. Isoflurane does not decompose in the presence of soda lime (at normal operating temperatures), and does not attack aluminum, tin, brass, iron or copper.
Pharmacodynamics
FORANE (isoflurane, USP) is an inhalation anesthetic. The MAC (minimum alveolar concentration) in man is as follows:
Induction of and recovery from isoflurane anesthesia are rapid. Isoflurane has a mild pungency, which limits the rate of induction, although excessive salivation or tracheobronchial secretions do not appear to be stimulated. Pharyngeal and laryngeal reflexes are readily obtunded. The level of anesthesia may be changed rapidly with isoflurane. Isoflurane is a profound respiratory depressant. RESPIRATION MUST BE MONITORED CLOSELY AND SUPPORTED WHEN NECESSARY. As anesthetic dose is increased, tidal volume decreases and respiratory rate is unchanged. This depression is partially reversed by surgical stimulation, even at deeper levels of anesthesia. Isoflurane evokes a sigh response reminiscent of that seen with diethyl ether and enflurane, although the frequency is less than with enflurane.
Blood pressure decreases with induction of anesthesia but returns toward normal with surgical stimulation. Progressive increases in depth of anesthesia produce corresponding decreases in blood pressure. Nitrous oxide diminishes the inspiratory concentration of isoflurane required to reach a desired level of anesthesia and may reduce the arterial hypotension seen with isoflurane alone. Heart rhythm is remarkably stable. With controlled ventilation and normal PaCO2, cardiac output is maintained despite increasing depth of anesthesia, primarily through an increase in heart rate, which compensates for a reduction in stroke volume. The hypercapnia, which attends spontaneous ventilation during isoflurane anesthesia further increases heart rate and raises cardiac output above awake levels. Isoflurane does not sensitize the myocardium to exogenously administered epinephrine in the dog. Limited data indicate that subcutaneous injection of 0.25 mg of epinephrine (50 mL of 1:200,000 solution) does not produce an increase in ventricular arrhythmias in patients anesthetized with isoflurane.
Muscle relaxation is often adequate for intra-abdominal operations at normal levels of anesthesia. Complete muscle paralysis can be attained with small doses of muscle relaxants. ALL COMMONLY USED MUSCLE RELAXANTS ARE MARKEDLY POTENTIATED WITH ISOFLURANE, THE EFFECT BEING MOST PROFOUND WITH THE NONDEPOLARIZING TYPE. Neostigmine reverses the effect of nondepolarizing muscle relaxants in the presence of isoflurane. All commonly used muscle relaxants are compatible with isoflurane.
Isoflurane can produce coronary vasodilation at the arteriolar level in selected animal models [1,2]; the drug is probably also a coronary dilator in humans. Isoflurane, like some other coronary arteriolar dilators, has been shown to divert blood from collateral dependent myocardium to normally perfused areas in an animal model (“coronary steal”) [3]. Clinical studies to date evaluating myocardial ischemia, infarction and death as outcome parameters have not established that the coronary arteriolar dilation property of isoflurane is associated with coronary steal or myocardial ischemia in patients with coronary artery disease [4,5,6,7].
Pharmacokinetics
Isoflurane undergoes minimal biotransformation in man. In the postanesthesia period, only 0.17% of the isoflurane taken up can be recovered as urinary metabolites.
Nonclinical Toxicology
There is limited information regarding Isoflurane Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Isoflurane Clinical Studies in the drug label.
How Supplied
FORANE (isoflurane, USP) is packaged in 100 mL and 250 mL amber-colored bottles.
FORANE (isoflurane, USP) is also supplied in the following aluminum bottles.
Storage
Store at room temperature 15°-30°C (59°-86°F).Isoflurane contains no additives and has been demonstrated to be stable at room temperature for periods in excess of five years.
Baxter and FORANE are trademarks of Baxter International Inc., registered in the United States Patent and Trademark Office.
Manufactured for Baxter Healthcare Corporation Deerfield, IL 60015 USA
For Product Inquiry 1 800 ANA DRUG (1-800-262-3784)
MLT 0159, C
Revised: July 2009
Images
Drug Images
{{#ask: Page Name::Isoflurane |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Isoflurane |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration.
Precautions with Alcohol
Alcohol-Isoflurane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Isoflurane Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Isoflurane Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Isoflurane |Label Name=Isoflurane label.png
}}