Template:Metoprolol: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 53: | Line 53: | ||
| melting_point = 120 | | melting_point = 120 | ||
}} | }} | ||
| tradename = Lopressor, Toprol-xl | |||
| Drugs.com = {{drugs.com|monograph|metoprolol-succinate}} | |||
| MedlinePlus = a682864 | |||
| licence_US = Metoprolol | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| legal_status = Rx-only | |||
| routes_of_administration = Oral, [[Intravenous|IV]] | |||
Revision as of 15:34, 21 March 2014
 | |
| Clinical data | |
|---|---|
| Trade names | Lopressor, Toprol-xl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Elimination half-life | 3-7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
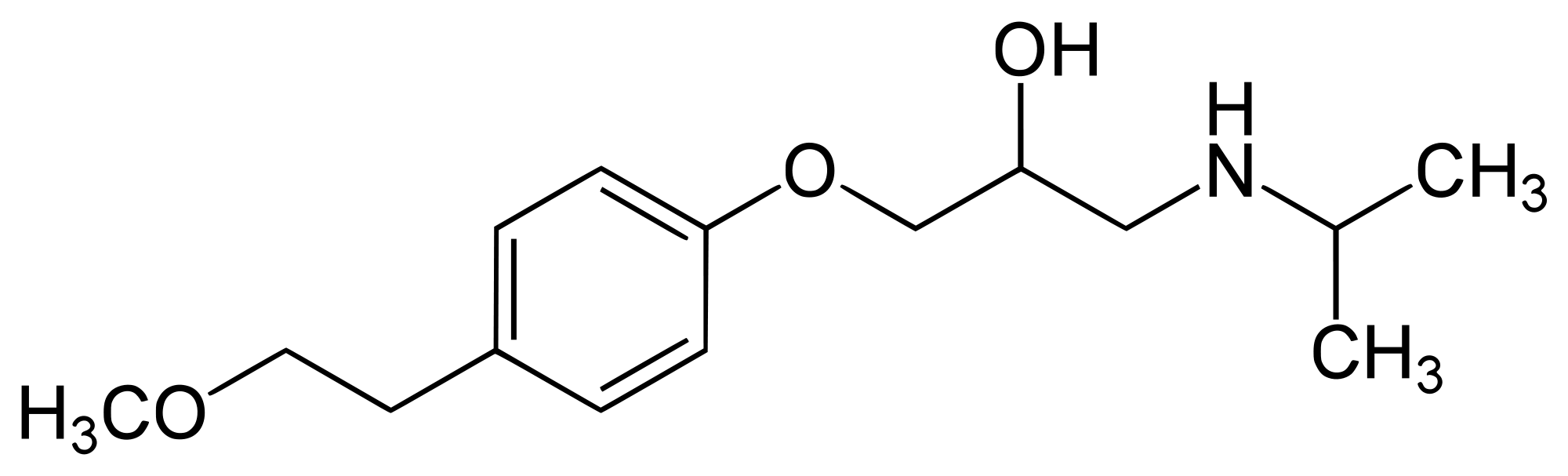
| Formula | C15H25NO3 |
| Molar mass | 267.364 g/mol |
| 3D model (JSmol) | |
| Melting point | 120 °C (248 °F) |
| |
| |
| (verify) | |
| tradename = Lopressor, Toprol-xl | Drugs.com = Monograph | MedlinePlus = a682864 | licence_US = Metoprolol | pregnancy_AU = C | pregnancy_US = C | legal_status = Rx-only | routes_of_administration = Oral, IV