Toprol XL patient counseling information: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with TOPROL-XL has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking TOPROL-XL. | Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with TOPROL-XL has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking TOPROL-XL. | ||
Heart failure patients should be advised to consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath. | Heart failure patients should be advised to consult their physician if they experience signs or symptoms of worsening [[heart failure]] such as [[weight gain]] or increasing [[shortness of breath]]. | ||
TOPROL-XL and PLENDIL are trademarks of the AstraZeneca group of companies.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPROL XL (METOPROLOL SUCCINATE) TABLET, EXTENDED RELEASE [BRYANT RANCH PREPACK] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=01038198-b4f0-41f3-9a9c-5c84e5a0d3b9 | publisher = | date = | accessdate = }}</ref> | TOPROL-XL and PLENDIL are trademarks of the AstraZeneca group of companies.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPROL XL (METOPROLOL SUCCINATE) TABLET, EXTENDED RELEASE [BRYANT RANCH PREPACK] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=01038198-b4f0-41f3-9a9c-5c84e5a0d3b9 | publisher = | date = | accessdate = }}</ref> | ||
Revision as of 00:30, 14 March 2014
 | |
| Clinical data | |
|---|---|
| Trade names | Lopressor, Toprol-xl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Elimination half-life | 3-7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
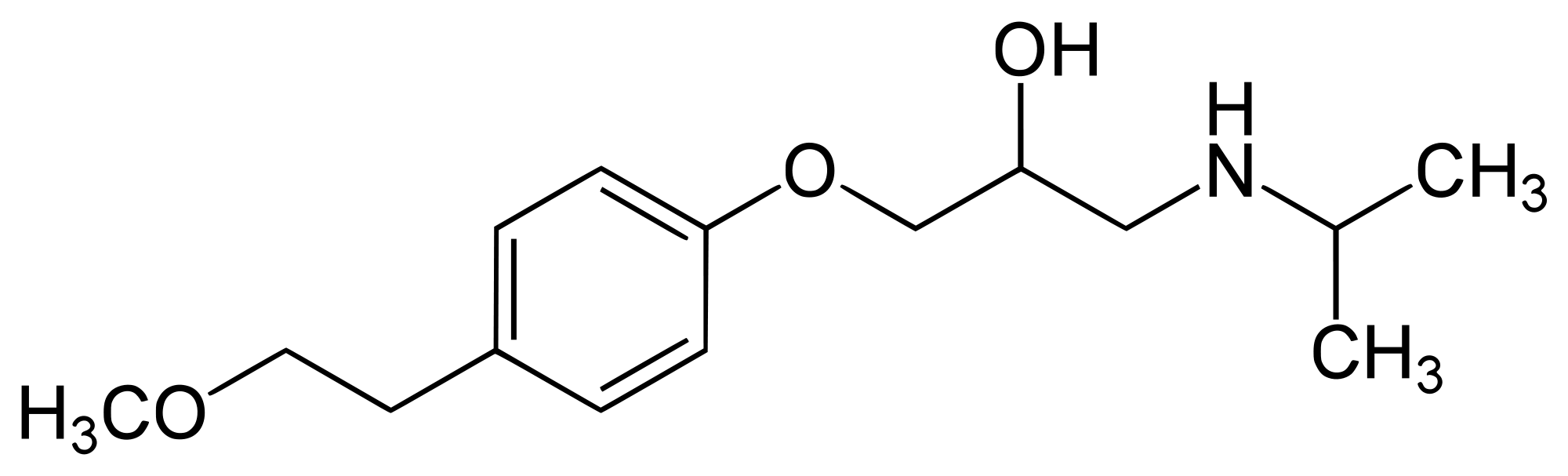
| Formula | C15H25NO3 |
| Molar mass | 267.364 g/mol |
| 3D model (JSmol) | |
| Melting point | 120 °C (248 °F) |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Advise patients to take TOPROL-XL regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the next scheduled dose (without doubling it). Patients should not interrupt or discontinue TOPROL-XL without consulting the physician.
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with TOPROL-XL has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking TOPROL-XL.
Heart failure patients should be advised to consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
TOPROL-XL and PLENDIL are trademarks of the AstraZeneca group of companies.[1]
References
Adapted from the FDA Package Insert.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Beta blockers
- Cardiovascular Drugs
- Drugs