Esmolol dosage and administration: Difference between revisions
Gerald Chi- (talk | contribs) |
Gerald Chi- (talk | contribs) |
||
| Line 57: | Line 57: | ||
The medication port is to be used solely for withdrawing an initial bolus from the bag. | The medication port is to be used solely for withdrawing an initial bolus from the bag. | ||
*Use aseptic technique when withdrawing the bolus dose. | |||
*Do not add any additional medications to the bag. | |||
{| | {| | ||
Revision as of 22:46, 20 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage And Administration
Dosing for the Treatment of Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia
BREVIBLOC is administered by continuous intravenous infusion with or without a loading dose. Additional loading doses and/or titration of the maintenance infusion (step-wise dosing) may be necessary based on desired ventricular response.
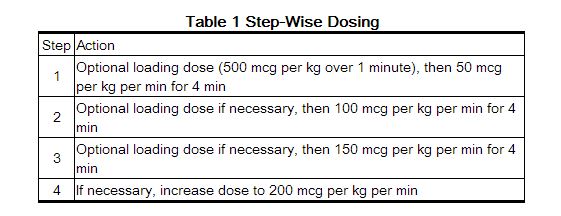 |
In the absence of loading doses, continuous infusion of a single concentration of esmolol reaches pharmacokinetic and pharmacodynamic steady-state in about 30 minutes.
The effective maintenance dose for continuous and step-wise dosing is 50 to 200 mcg per kg per minute, although doses as low as 25 mcg per kg per minute have been adequate. Dosages greater than 200 mcg per kg per minute provide little added heart rate lowering effect, and the rate of adverse reactions increases.
Maintenance infusions may be continued for up to 48 hours.
Intraoperative and Postoperative Tachycardia and Hypertension
In this setting it is not always advisable to slowly titrate to a therapeutic effect. Therefore two dosing options are presented: immediate control and gradual control.
Immediate Control
Administer 1 mg per kg as a bolus dose over 30 seconds followed by an infusion of 150 mcg per kg per min if necessary. Adjust the infusion rate as required to maintain desired heart rate and blood pressure. Refer to Maximum Recommended Doses below.
Gradual Control
Administer 500 mcg per kg as a bolus dose over 1 minute followed by a maintenance infusion of 50 mcg per kg per min for 4 minutes. Depending on the response obtained, continue dosing as outlined for supraventricular tachycardia. Refer to Maximum Recommended Doses below.
Maximum Recommended Doses
For the treatment of tachycardia, maintenance infusion dosages greater than 200 mcg per kg per min are not recommended; dosages greater than 200 mcg per kg per min provide little additional heart rate-lowering effect, and the rate of adverse reactions increases. For the treatment of hypertension, higher maintenance infusion dosages (250-300 mcg per kg per min) may be required. The safety of doses above 300 mcg per kg per minute has not been studied.
Transition from BREVIBLOC Injection Therapy to Alternative Drugs
After patients achieve adequate control of the heart rate and a stable clinical status, transition to alternative antiarrhythmic drugs may be accomplished.
When transitioning from BREVIBLOC to alternative drugs, the physician should carefully consider the labeling instructions of the alternative drug selected and reduce the dosage of BREVIBLOC as follows:
Thirty minutes following the first dose of the alternative drug, reduce the BREVIBLOC infusion rate by one-half (50%). After administration of the second dose of the alternative drug, monitor the patient’s response and if satisfactory control is maintained for the first hour, discontinue the BREVIBLOC infusion.
Directions for Use
- BREVIBLOC injection is available in a pre-mixed bag and ready-to-use vial. BREVIBLOC is not compatible with Sodium Bicarbonate (5%) solution (limited stability) or furosemide (precipitation).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
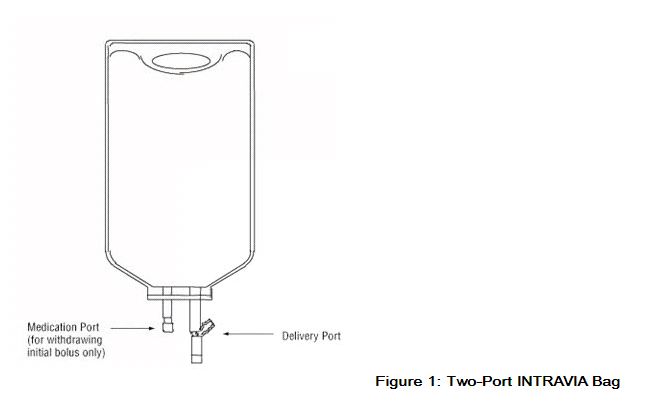
- Premixed Bag
The medication port is to be used solely for withdrawing an initial bolus from the bag.
- Use aseptic technique when withdrawing the bolus dose.
- Do not add any additional medications to the bag.
 |
Ready-to-Use Vial:
The Ready-to-use Vial may be used to administer a loading dosage by hand-held syringe while the maintenance infusion is being prepared
Compatibility with Commonly Used Intravenous Fluids
BREVIBLOC was tested for compatibility with ten commonly used intravenous fluids at a final concentration of 10 mg esmolol hydrochloride per mL. BREVIBLOC was found to be compatible with the following solutions and was stable for at least 24 hours at controlled room temperature or under refrigeration:[1]
- Dextrose (5%) Injection, USP
- Dextrose (5%) in Lactated Ringer’s Injection
- Dextrose (5%) in Ringer’s Injection
- Dextrose (5%) and Sodium Chloride (0.45%) Injection, USP
- Dextrose (5%) and Sodium Chloride (0.9%) Injection, USP
- Lactated Ringer’s Injection, USP
- Potassium Chloride (40 mEq/liter) in Dextrose (5%) Injection, USP
- Sodium Chloride (0.45%) Injection, USP
- Sodium Chloride (0.9%) Injection, USP
References
Adapted from the FDA Package Insert.