Benznidazole: Difference between revisions
No edit summary |
No edit summary |
||
| Line 244: | Line 244: | ||
(Description) | (Description) | ||
|drugBox | |drugBox= | ||
{{Drugbox2 | |||
| verifiedrevid = 414638558 | |||
| IUPAC_name = ''N''-benzyl-2-(2-nitro-1''H''-imidazol-1-yl)acetamide | |||
| image = Benznidazole_Pharmocology_Box_Image.png | |||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = Rochagan, Radanil<ref name=JAMA2007/> | ||
| | | Drugs.com = {{drugs.com|CONS|benznidazole}} | ||
| | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| | | pregnancy_category = | ||
| legal_status = | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | ||
| routes_of_administration = | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = by mouth | |||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = | | bioavailability = High | ||
| metabolism = | | protein_bound = | ||
| elimination_half-life = | | metabolism = [[Liver]] | ||
| excretion = | | elimination_half-life = 12 hours | ||
| excretion = [[Kidney]] and fecal | |||
<!--Identifiers--> | <!--Identifiers--> | ||
| CAS_number_Ref = | | CAS_number_Ref = {{cascite|correct|CAS}} | ||
| CAS_number = | | CAS_number = 22994-85-0 | ||
| ATC_prefix = | | ATC_prefix = P01 | ||
| ATC_suffix = | | ATC_suffix = CA02 | ||
| PubChem = | | PubChem = 31593 | ||
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | | DrugBank = | ||
| DrugBank = | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ChemSpiderID_Ref = | | ChemSpiderID = 29299 | ||
| ChemSpiderID = | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| UNII_Ref = | | UNII = YC42NRJ1ZD | ||
| UNII = | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| KEGG_Ref = | | KEGG = D02489 | ||
| KEGG = | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| ChEBI_Ref = | | ChEBI = 133833 | ||
| ChEBI = | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ChEMBL_Ref = | | ChEMBL = 110 | ||
| ChEMBL = | |||
<!--Chemical data--> | <!--Chemical data--> | ||
| C= | H= | N= | O= | | C=12 | H=12 | ||
| molecular_weight = | | N=4 | O=3 | ||
| smiles = | | molecular_weight = 260.249 g/mol | ||
| smiles = O=[N+]([O-])c1nccn1CC(=O)NCc2ccccc2 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI_Ref = | | StdInChI = 1S/C12H12N4O3/c17-11(14-8-10-4-2-1-3-5-10)9-15-7-6-13-12(15)16(18)19/h1-7H,8-9H2,(H,14,17) | ||
| StdInChI = | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey_Ref = | | StdInChIKey = CULUWZNBISUWAS-UHFFFAOYSA-N | ||
| StdInChIKey = | | melting_point = 188.5 | ||
| melting_point = | | melting_high = 190 | ||
}} | }} | ||
|mechAction=*Benznidazole is a nitroimidazole antimicrobial drug. | |mechAction=*Benznidazole is a nitroimidazole antimicrobial drug. | ||
|structure= | |structure=[[image:Benznidazole_Chemical_Structure.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
|PD=*The pharmacodynamics of benznidazole is unknown. | |PD=*The pharmacodynamics of benznidazole is unknown. | ||
Revision as of 16:32, 29 June 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Benznidazole is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Benznidazole in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Benznidazole and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
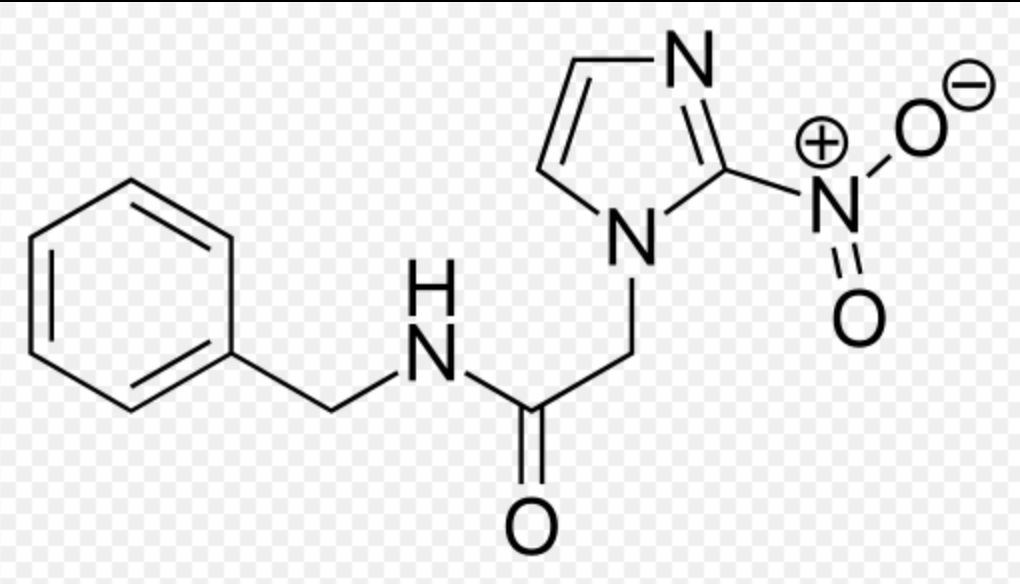
| |
Benznidazole
| |
| Systematic (IUPAC) name | |
| N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide | |
| Identifiers | |
| CAS number | |
| ATC code | P01 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 260.249 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 188.5–190 °C (371–374 °F) |
| Pharmacokinetic data | |
| Bioavailability | High |
| Metabolism | Liver |
| Half life | 12 hours |
| Excretion | Kidney and fecal |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | by mouth |
Mechanism of Action
- Benznidazole is a nitroimidazole antimicrobial drug.
Structure

Pharmacodynamics
- The pharmacodynamics of benznidazole is unknown.
Pharmacokinetics
Absorption
- The absorption of benznidazole from three different 100 mg benznidazole preparations was comparable when administered as a single dose under fasting conditions in adult healthy volunteers (TABLE 6).

Effect of Food
- Benznidazole Cmax and AUC were not affected by the administration of Benznidazole 100 mg tablet with a high-fat, high-caloric meal (approximately 1034 total kcal, 67 kcal from fat, 42 kcal from carbohydrates, 59 kcal from protein) compared with fasted conditions in adult healthy volunteers. Serum concentrations of benznidazole reached peak levels at 3.2 hours (1-10 hours) after administration of Benznidazole Tablets 100 mg tablet after a high-fat, high-caloric meal, and at 2.0 hours (0.5-4 hours) in fasted conditions.
Distribution
- Protein binding is reported to be approximately 44 to 60 %.
Elimination
- The elimination half-life on benznidazole is approximately 13 hours in healthy volunteers following single dose.
Metabolism
- Benznidazole metabolism pathway is unknown.
Excretion
- Benznidazole and unknown metabolites are reported to be excreted in the urine and feces.
Specific Populations
- The effect of sex, race, renal impairment, or hepatic impairment on the pharmacokinetics of benznidazole is unknown.
Drug Interaction Studies
- In vitro studies showed that benznidazole is a P-gp substrate and does not notably induce Cytochrome P450 enzymes 1A2, 2B6, and 3A4 at concentrations up to 100 uM.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
- Long-term carcinogenicity studies for benznidazole have not been performed.
- Nitroimidazoles, which have similar chemical structures to benznidazole have been reported to be carcinogenic in mice and rats.
Genetic Toxicity
- Genotoxicity of benznidazole has been demonstrated in vitro in several bacterial species and mammalian cell systems and in vivo in mammals.
- Benznidazole was mutagenic in several strains of S. typhimurium (TA 100, 102 1535, 1537, 1538, 97, 98 99 53 and UTH8414), E.coli, and K. pneumoniae.
- Benznidazole was genotoxic in several in vitro mammalian cell assays including a chromosome aberration assay in human lymphocytes and in sister chromatid exchange assays in human lymphocytes and in Human Hep G2 cells.
- In vivo, benznidazole was shown to be positive for genotoxicity in a mouse bone marrow micronucleus assay, in mouse and human red blood cell micronucleus assays, in a mouse abnormal sperm head assay and in a human peripheral blood lymphocyte assay. However in other micronucleus studies in mice and rats, oral doses of benznidazole did not cause a significant increase in the frequency of chromosomal aberrations in bone marrow cells or micronuclei in peripheral blood cells.
Impairment of Fertility
- In a 6-month, chronic repeated-dosing study with Wistar rats, benznidazole was shown to produce dose-dependent testicular and epididymal atrophy at a dose of 30 mg/kg/day (approximately equivalent to 0.6 times the MRHD based on whole body surface area comparisons). Aspermia was also evident in affected rats, but fertility was not assessed in this study. The NOAEL value in this study was considered to be 10 mg/kg/day (5 mg/kg twice daily) in males which is approximately 0.2-times the MRHD based on body surface area comparison. In other literature reports, benznidazole has been shown to cause testicular atrophy and inhibit spermatogenesis in pubertal and adult rats and mice5-7.
- In a female fertility study, oral (gavage) administration of benznidazole to female Wistar rats twice daily for a 2-week pre-mating period, during mating, and through day 7 of gestation was associated with transient lower body weight gain and food consumption. There was no benznidazole-related effect on mating performance or fertility and no adverse macroscopic or reproductive organ weight changes. However, benznidazole reproductive performance was associated with a higher post-implantation loss with lower live litter size at a dose of 150 mg/kg/day (equivalent to approximately 3 times the MRHD based on whole body surface area comparisons). The NOAEL value for this study was consider to be 50 mg/kg/day which is approximately equivalent to the MRHD based on whole body surface area comparison.
Animal Toxicology and/or Pharmacology
- Single oral dose toxicity studies in rats have established that benznidazole causes ultrastructural changes in the adrenal cortex, colon, esophagus, ovaries, and testis 5, 8-11. In these tissues, these changes were associated with the presences of nitro reductase activity, the production of reactive metabolites, and or covalent binding of metabolites.
- Neurotoxicity including brain axonal degeneration and Purkinje cell degeneration was observed with repeated-oral dosing in dogs without adverse changes in peripheral nerves12-14. Neurological signs included: apathy, hypertonia, hyperreflexia, ataxia, loss of balance, oscillatory movements of the trunk and head, strong contractions of the back and leg muscles, opisthotonus and nystagmus. Neurotoxicity was not observed in other test species, including mouse, rat, guinea pig, and rabbit.
Clinical Studies
- The safety and effectiveness of benznidazole for the treatment of Chagas disease in patients 6 to 12 years of age was established in two adequate and well-controlled trials (Trial 1 and Trial 2) as described below.
- Trial 1 was a randomized, double-blind, placebo-controlled trial in children 6 to 12 years of age with chronic indeterminate Chagas disease conducted in Argentina. The chronic indeterminate form of Chagas disease includes patients with serologic evidence of T. cruzi infection without symptoms of cardiac or gastrointestinal disease. A total of 106 patients were randomized to receive either benznidazole (5 mg/kg/day for 60 days) or placebo and followed for 4 years. Patients with at least two positive conventional serologic tests for antibodies to T. cruzi were included in the study. The conventional serologic tests used include indirect hemagglutination assay (IHA), immunofluorescence antibody assay (IFA), and/or enzyme linked immunosorbent assay (ELISA) and were based on the detection of antibodies against T. cruzi parasites.
- Trial 2 was a randomized, double-blind, placebo-controlled trial in pediatric patients 7 to 12 years of age with chronic indeterminate Chagas disease conducted in Brazil. A total of 129 patients were randomized to receive either benznidazole (7.5 mg/kg/day for 60 days) or placebo and followed for 3 years. Patients with three positive conventional serologic tests for antibodies to T. cruzi were included in the study. The conventional serologic tests include IHA, IFA, and/or ELISA and were based on the detection of antibodies against T. cruzi parasites.
- Both trials measured antibodies by conventional and nonconventional assays. The nonconventional assays include F29-ELISA and AT- chemiluminescence-ELISA that are based on detection of anti-T. cruzi IgG antibodies against the recombinant antigens, F29 and AT from the flagella of T. cruzi parasites. Benznidazole treatment resulted in a significantly higher percentage of seronegative patients by a nonconventional assay. Results at the end of follow-up are reported in the following table.

- In Trial 1 using conventional ELISA, 4 of 53 (7.5%) benznidazole subjects and 2 of 50 (4.0%) placebo subjects seroconverted to negative by the end of follow-up (difference 3.5, 95% CI (-7.0, 14.9)). In Trial 2 using conventional ELISA, 4 of 64 (6.3%) of benznidazole subjects and 0 of 65 placebo subjects seroconverted to negative by the end of follow-up (difference 6.3, 95% CI (0.3, 15.2)).
How Supplied
- Benznidazole Tablets (12.5 mg or 100 mg) are supplied as follows:
- 100 mg white tablets, round and functionally scored twice as a cross on both sides. Each tablet is about 10 mm in diameter debossed with “E” on one side of each quarter portion.
- 12.5 mg white tablets, round and unscored. Each tablet is about 5 mm in diameter debossed with “E” on one side.
- Benznidazole Tablets 100 mg are available in bottles of 100 tablets (NDC 0642-7464-10).
- Benznidazole Tablets 12.5 mg are available in bottles of 100 tablets (NDC 0642-7463-12).
Storage
- Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Keep bottle tightly closed and protect from moisture.
Images
Drug Images
{{#ask: Page Name::Benznidazole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Benznidazole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Embryo-Fetal Toxicity
- Advise pregnant women and females of reproductive potential that exposure to Benznidazole Tablets during pregnancy can result in fetal harm.
- Advise females to inform their healthcare provider of a known or suspected pregnancy.
- Advise females of reproductive potential to use effective contraception while taking Benznidazole Tablets and for 5 days after the last dose.
Lactation
- Advise women not to breastfeed during treatment with Benznidazole Tablets.
Infertility
- Advise males of reproductive potential that Benznidazole Tablets may impair fertility.
Important Administration Instructions
- Advise patients and parents/caregivers of pediatric patients taking Benznidazole Tablets that:
- Benznidazole Tablets 100 mg are functionally scored tablets which can be split into one-half (50 mg) or one-quarter (25 mg) at the scored lines to provide doses less than 100 mg.
- Benznidazole Tablets 12.5 mg and 100 mg (whole or split) can be made into a slurry in a specified volume of water for the pediatric population.
Hypersensitivity Skin Reactions
- Advise patients that serious skin reactions can occur with Benznidazole Tablets. In case of skin reactions, presenting with additional symptoms of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is necessary.
Central and Peripheral Nervous System Effects
- Advise patients that treatment can potentially cause paresthesia or symptoms of peripheral neuropathy. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended.
Hematological Manifestations of Bone Marrow Depression
- Advise patients that there have been hematological manifestations of bone marrow depression, such as anemia and leukopenia, which are reversible, and normalized after treatment discontinuation.
Interaction with Alcohol
- Advise patients to discontinue consumption of alcoholic beverages or products containing propylene glycol while taking Benznidazole Tablets and for at least three days afterward because abdominal cramps, nausea, vomiting, headaches, and flushing may occur.
Precautions with Alcohol
Alcohol-Benznidazole interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Benznidazole Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Benznidazole Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.