Rimabotulinumtoxinb: Difference between revisions
No edit summary |
No edit summary |
||
| Line 85: | Line 85: | ||
MYOBLOC is available in the following three presentations. | MYOBLOC is available in the following three presentations. | ||
[[File:rimabotulinumtoxinb2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:rimabotulinumtoxinb2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|storage=*Store under refrigeration at 2°- 8°C (36°- 46°F). | |storage=*Store under refrigeration at 2°- 8°C (36°- 46°F). | ||
| Line 94: | Line 92: | ||
*Ready to use; no reconstitution required. The recommended storage condition for MYOBLOC is refrigeration at 2°-8°C. | *Ready to use; no reconstitution required. The recommended storage condition for MYOBLOC is refrigeration at 2°-8°C. | ||
|packLabel=[[File:Rimabotulinumtoxinb3.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Rimabotulinumtoxinb4.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Rimabotulinumtoxinb5.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Rimabotulinumtoxinb6.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=The physician should provide a copy of the FDA-Approved Patient Medication Guide and review the contents with the patient. Patients should be advised to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking or breathing), or if any existing symptom worsens. | |fdaPatientInfo=The physician should provide a copy of the FDA-Approved Patient Medication Guide and review the contents with the patient. Patients should be advised to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking or breathing), or if any existing symptom worsens. | ||
Revision as of 14:25, 27 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
DISTANT SPREAD OF TOXIN EFFECT
See full prescribing information for complete Boxed Warning.
Postmarketing reports indicate that the effects of MYOBLOC and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, cases of spread of effect have occurred at doses comparable to those used to treat cervical dystonia and at lower doses.
|
Overview
Rimabotulinumtoxinb is a neurotoxic protein that is FDA approved for the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dry mouth, dysphagia, dyspepsia, and injection site pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- MYOBLOC is indicated for the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia.
Dosage
The recommended initial dose of MYOBLOC for patients with a prior history of tolerating botulinum toxin injections is 2,500 to 5,000 Units divided among affected muscles (see CLINICAL STUDIES). Patients without a prior history of tolerating botulinum toxin injections should receive a lower initial dose. Subsequent dosing should be optimized according to the patient's individual response. MYOBLOC should be administered by physicians familiar and experienced in the assessment and management of patients with CD.
The method described for performing the potency assay is specific to Solstice Neurosciences' manufacture of MYOBLOC. Due to differences in the specific details of this assay such as the vehicle, dilution scheme and laboratory protocols for various potency assays, Units of biological activity of MYOBLOC cannot be compared to or converted into units of any other botulinum toxin or any toxin assessed with any other specific assay method. Therefore, differences in species' sensitivities to different botulinum neurotoxin serotypes preclude extrapolation of animal dose-activity relationships to human dose estimates.
The duration of effect in patients responding to MYOBLOC treatment has been observed in studies to be between 12 and 16 weeks at doses of 5,000 Units or 10,000 Units (see CLINICAL STUDIES).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rimabotulinumtoxinb in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rimabotulinumtoxinb in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rimabotulinumtoxinb in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rimabotulinumtoxinb in pediatric patients.
Contraindications
MYOBLOC is contraindicated in patients with a known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
MYOBLOC is contraindicated for use in patients with infection at the proposed injection site(s).
Warnings
|
DISTANT SPREAD OF TOXIN EFFECT
See full prescribing information for complete Boxed Warning.
Postmarketing reports indicate that the effects of MYOBLOC and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, cases of spread of effect have occurred at doses comparable to those used to treat cervical dystonia and at lower doses.
|
Lack of Interchangeability between Botulinum Toxin Products The potency Units of MYOBLOC are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of MYOBLOC cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method (see DESCRIPTION).
Spread of Toxin Effect Postmarketing safety data from MYOBLOC and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, and particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than doses used to treat cervical dystonia.
Dysphagia and Breathing Difficulties in Treatment of Cervical Dystonia Treatment with MYOBLOC and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved.
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several months, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment of cervical dystonia with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been postmarketing reports of serious breathing difficulties, including respiratory failure, in cervical dystonia patients. Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin (see ADVERSE REACTIONS, CLINICAL PHARMACOLOGY).
Pre-Existing Neuromuscular Disorders Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of MYOBLOC (see ADVERSE REACTIONS).
Human Albumin This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Precations
Only 9 subjects without a prior history of tolerating injections of type A botulinum toxin have been studied. Treatment of botulinum toxin naïve patients should be initiated at lower doses of MYOBLOC (see ADVERSE REACTIONS: OVERVIEW).
Adverse Reactions
Clinical Trials Experience
Overview The most commonly reported adverse events associated with MYOBLOC treatment in all studies were dry mouth, dysphagia, dyspepsia, and injection site pain. Dry mouth and dysphagia were the adverse reactions most frequently resulting in discontinuation of treatment. There was an increased incidence of dysphagia with increased dose in the sternocleidomastoid muscle. The incidence of dry mouth showed some dose-related increase with doses injected into the splenius capitis, trapezius and sternocleidomastoid muscles.
Only nine subjects without a prior history of tolerating injections of type A botulinum toxin have been studied. Adverse event rates have not been adequately evaluated in these patients, and may be higher than those described in Table 3.
Discussion Adverse reaction rates observed in the clinical trials for a product cannot be directly compared to rates in clinical trials for another product and may not reflect the rates observed in actual clinical practice. However, adverse reaction information from clinical trials does provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
MYOBLOC was studied in both placebo controlled single treatment studies and uncontrolled repeated treatment studies; most treatment sessions and patients were in the uncontrolled studies. The data described below reflect exposure to MYOBLOC at varying doses in 570 subjects, including more than 300 patients with 4 or more treatment sessions. Most treatment sessions were at doses of 12,500 Units or less. There were 57 patients administered a dose of 20,000 or 25,000 Units. All but nine patients had a prior history of receiving type A botulinum toxin and adequately tolerating the treatment to have received repeated doses.
The rates of adverse events and association with MYOBLOC are best assessed in the results from the placebo controlled studies of a single treatment session with active monitoring. The data in Table 3 reflect those adverse events occurring in at least 5% of patients exposed to MYOBLOC treatment in pooled placebo controlled clinical trials. Annual rates of adverse events are higher in the overall data which includes longer duration follow-up of patients with repeated treatment experience. The mean age of the population in these studies was 55-years-old with approximately 66% being female. Most of the patients studied were Caucasian and all had cervical dystonia that was rated as moderate to severe in severity.

In the overall clinical trial experience with MYOBLOC (570 patients, including the uncontrolled studies), most cases of dry mouth or dysphagia were reported as mild or moderate in severity. Severe dysphagia was reported by 3% of patients. Severe dry mouth was reported by 6% of patients. Dysphagia and dry mouth were the most frequent adverse events reported as a reason for discontinuation from repeated treatment studies. These adverse events led to discontinuation from further treatments with MYOBLOC in some patients even when not reported as severe.
The following additional adverse events were reported in 2% or greater of patients participating in any of the clinical studies (COSTART terms, by body system):
Body as a Whole: allergic reaction, fever, headache related to injection, chest pain, chills, hernia, malaise, abscess, cyst, neoplasm, viral infection; Musculoskeletal: arthritis, joint disorder; Cardiovascular System: migraine; Respiratory: dyspnea, lung disorder, pneumonia; Nervous System: anxiety, tremor, hyperesthesia, somnolence, confusion, pain related to CD/torticollis, vertigo, vasodilation; Digestive System: gastrointestinal disorder, vomiting, glossitis, stomatitis, tooth disorder; Skin and Appendages: pruritis; Urogenital System: urinary tract infection, cystitis, vaginal moniliasis; Special Senses: amblyopia, otitis media, abnormal vision, taste perversion, tinnitus; Metabolic and Nutritional Disorders: peripheral edema, edema, hypercholesterolemia; Hemic and Lymphatic System: ecchymosis.
Postmarketing Experience
The following adverse events have been reported during postmarketing use for approved and unapproved indications: angioedema, urticaria, rash and constipation.
Drug Interactions
Co-administration of MYOBLOC and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated.
The effect of administering different botulinum neurotoxin serotypes at the same time or within less than 4 months of each other is unknown. However, neuromuscular paralysis may be potentiated by co-administration or overlapping administration of different botulinum toxin serotypes.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with MYOBLOC. It is also not known whether MYOBLOC can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. MYOBLOC should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rimabotulinumtoxinb in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rimabotulinumtoxinb during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when MYOBLOC is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
In the controlled studies summarized in CLINICAL STUDIES, for MYOBLOC treated patients, 152 (74.5%) were under the age of 65, and 52 (25.5%) were aged 65 or greater. For these age groups, the most frequent reported adverse events occurred at similar rates in both age groups. Efficacy results did not suggest any large differences between these age groups.
Very few patients aged 75 or greater were enrolled, therefore no conclusions regarding the safety and efficacy of MYOBLOC within this age group can be determined.
Gender
There is no FDA guidance on the use of Rimabotulinumtoxinb with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rimabotulinumtoxinb with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rimabotulinumtoxinb in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rimabotulinumtoxinb in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rimabotulinumtoxinb in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rimabotulinumtoxinb in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Rimabotulinumtoxinb Administration in the drug label.
Monitoring
There is limited information regarding Rimabotulinumtoxinb Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Rimabotulinumtoxinb and IV administrations.
Overdosage
Excessive doses of MYOBLOC may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis (see WARNINGS and PRECAUTIONS).Symptomatic treatment may be necessary.
Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur, the person should be medically supervised for several weeks for signs and symptoms of excessive muscle weakness or muscle paralysis.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC.
Pharmacology
There is limited information regarding Rimabotulinumtoxinb Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Rimabotulinumtoxinb Mechanism of Action in the drug label.
Structure
There is limited information regarding Rimabotulinumtoxinb Structure in the drug label.
Pharmacodynamics
There is limited information regarding Rimabotulinumtoxinb Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Rimabotulinumtoxinb Pharmacokinetics in the drug label.
Nonclinical Toxicology
No long-term carcinogenicity studies in animals have been performed.
Clinical Studies
There is limited information regarding Rimabotulinumtoxinb Clinical Studies in the drug label.
How Supplied
MYOBLOC is provided as a clear and colorless to light-yellow sterile injectable solution in single-use 3.5-mL glass vials. Each single-use vial of formulated MYOBLOC contains 5,000 Units1 of botulinum toxin type B per milliliter in 0.05% human serum albumin, 0.01 M sodium succinate, 0.1 M sodium chloride at approximately pH 5.6.
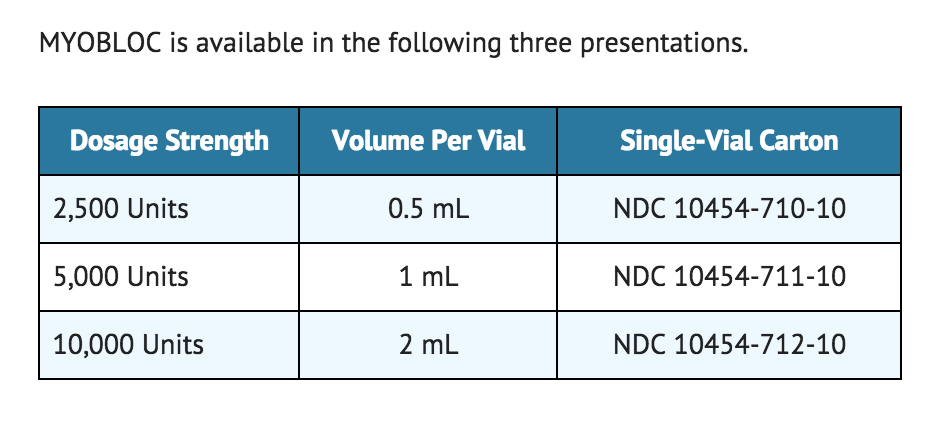
MYOBLOC is available in the following three presentations.
Storage
- Store under refrigeration at 2°- 8°C (36°- 46°F).
- DO NOT FREEZE. DO NOT SHAKE.
- Protect from light. No U.S. Standard of Potency.
- Ready to use; no reconstitution required. The recommended storage condition for MYOBLOC is refrigeration at 2°-8°C.
Images
Drug Images
{{#ask: Page Name::Rimabotulinumtoxinb |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Rimabotulinumtoxinb |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
The physician should provide a copy of the FDA-Approved Patient Medication Guide and review the contents with the patient. Patients should be advised to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking or breathing), or if any existing symptom worsens.
Patients should be counseled that if loss of strength, muscle weakness, or impaired vision occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Precautions with Alcohol
Alcohol-Rimabotulinumtoxinb interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Rimabotulinumtoxinb Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Rimabotulinumtoxinb Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.