Methscopolamine bromide: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |indicationType=treatment |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<i><span style="color:#FF0000...") |
No edit summary |
||
| Line 7: | Line 7: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Methscopolamine bromide in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Methscopolamine bromide in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Methscopolamine bromide in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Methscopolamine bromide in pediatric patients. | ||
|contraindications=Glaucoma; obstructive uropathy (e.g., bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (e.g., pyloroduodenal stenosis); paralytic ileus; intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis. | |||
Methscopolamine Bromide 2.5 mg Tablets/ Methscopolamine Bromide 5 mg Tablets are contraindicated in patients who are hypersensitive to methscopolamine bromide or related drugs. | |||
|warnings=In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with drug use. | |||
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful. | |||
Methscopolamine bromide may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug. | |||
With overdosage, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis. | |||
===Precautions=== | |||
1. General precautions: Use Methscopolamine Bromide 2.5 mg Tablets/Methscopolamine Bromide 5 mg Tablets with caution in the elderly and in all patients with: autonomic neuropathy; hepatic or renal disease; or ulcerative colitis-large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate "toxic megacolon," a serious complication of the disease. | |||
The drug also should be used with caution in patients having hyperthyroidism, coronary heart disease, congestive heart failure, tachyrhythmia, tachycardia, hypertension, or prostatic hypertrophy. | |||
2. Information for patient: See statement under WARNINGS. | |||
3. Laboratory tests: Progress of the peptic ulcer under treatment should be followed by upper gastrointestinal contrast radiology or endoscopy to insure healing. Stool tests for occult blood and blood hemoglobin or hematocrit values should be followed to rule out bleeding from the ulcer. | |||
|clinicalTrials=The following-adverse reactions have been observed, but there is not enough data to support an estimate of frequency. | |||
Cardiovascular: Tachycardia, palpitation. | |||
Allergic: Severe allergic reaction or drug idiosyncrasies including anaphylaxis. | |||
CNS: Headaches, nervousness, mental confusion, drowsiness, dizziness. | |||
Special Senses: Blurred vision, dilation of the pupil, cycloplegia, increased ocular tension, loss of taste. | |||
Renal: Urinary hesitancy and retention. | |||
Gastrointestinal: Nausea, vomiting, constipation, bloated feeling. | |||
Dermatologic: Decreased sweating, urticaria and other dermal manifestations. | |||
Miscellaneous: Xerostomia, weakness, insomnia, impotence, suppression of lactation. | |||
|drugInteractions=Additive anticholinergic effects may result from concomitant use with antipsychotics, tricyclic antidepressants, and other drugs with anticholinergic effects. Concomitant administration with antacids may interfere with the absorption of methscopolamine bromide. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Animal reproduction studies have not been conducted with methscopolamine bromide. It also is not known whether methscopolamine bromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methscopolamine bromide should be given to a pregnant woman only if clearly needed. | |||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methscopolamine bromide is administered to a nursing woman. | |||
|useInPed=Safety and efficacy in children have not been established. | |||
|overdose=The symptoms of overdosage with Methscopolamine Bromide 2.5 mg Tablets/Methscopolamine Bromide 5 mg Tablets progress from intensification of the usual side effects to CNS disturbances (from restlessness and excitement to psychotic behavior), circulatory changes (flushing, fall in blood pressure, circulatory failure), respiratory failure, paralysis, and coma. | |||
Measures to be taken are (1) induction of emesis and (2) injection of physostigmine 0.5 to 2 mg intravenously, and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (alcohol sponging, ice packs). Excitement of a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 ml of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns. | |||
The oral LD50 in rats is 1,352 to 2,617 mg/kg. | |||
No data is available on the dialyzability of methscopolamine bromide. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 442016426 | |||
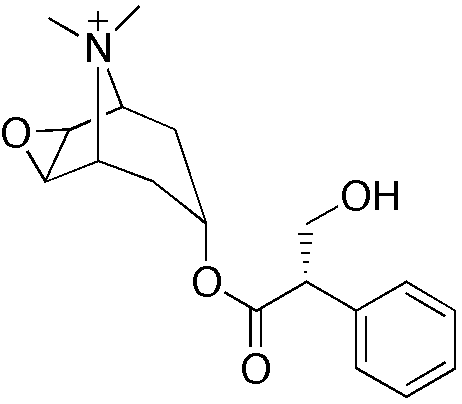
| IUPAC_name = (1''R'',2''S'',4''R'',5''S'',7''R'')-{[(2''R'')-3-hydroxy-2-phenylpropanoyl]oxy}-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.0<sup>2,4</sup>]nonane | |||
| image = Methscopolamine.png | |||
<!--Clinical data--> | |||
| tradename = Pamine, Extendryl, AlleRx, Rescon | |||
| Drugs.com = {{drugs.com|monograph|methscopolamine-bromide}} | |||
| MedlinePlus = a606008 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = 3–4 hrs | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number = 155-41-9 | |||
| ATC_prefix = A03 | |||
| ATC_suffix = BB03 | |||
| ATC_supplemental = {{ATC|S01|FA03}} | |||
| PubChem = 441342 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00462 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 21106347 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = RTN51LK7WL | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 376897 | |||
<!--Chemical data--> | |||
| C=18 | H=24 | N=1 | O=4 | |||
| molecular_weight = 318.388 g/mol | |||
| smiles = OC[C@H](c1ccccc1)C(=O)O[C@H]2C[C@@H]3[N+](C)(C)[C@H](C2)[C@H]4O[C@@H]34 | |||
| InChI = 1/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | |||
| InChIKey = LZCOQTDXKCNBEE-IKIFYQGPBD | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = LZCOQTDXKCNBEE-IKIFYQGPSA-N | |||
}} | |||
|alcohol=Alcohol-Methscopolamine bromide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Methscopolamine bromide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 18:13, 21 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Methscopolamine bromide is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Methscopolamine bromide FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methscopolamine bromide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methscopolamine bromide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Methscopolamine bromide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methscopolamine bromide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methscopolamine bromide in pediatric patients.
Contraindications
Glaucoma; obstructive uropathy (e.g., bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (e.g., pyloroduodenal stenosis); paralytic ileus; intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
Methscopolamine Bromide 2.5 mg Tablets/ Methscopolamine Bromide 5 mg Tablets are contraindicated in patients who are hypersensitive to methscopolamine bromide or related drugs.
Warnings
In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with drug use.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Methscopolamine bromide may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
With overdosage, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis.
Precautions
1. General precautions: Use Methscopolamine Bromide 2.5 mg Tablets/Methscopolamine Bromide 5 mg Tablets with caution in the elderly and in all patients with: autonomic neuropathy; hepatic or renal disease; or ulcerative colitis-large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate "toxic megacolon," a serious complication of the disease.
The drug also should be used with caution in patients having hyperthyroidism, coronary heart disease, congestive heart failure, tachyrhythmia, tachycardia, hypertension, or prostatic hypertrophy.
2. Information for patient: See statement under WARNINGS.
3. Laboratory tests: Progress of the peptic ulcer under treatment should be followed by upper gastrointestinal contrast radiology or endoscopy to insure healing. Stool tests for occult blood and blood hemoglobin or hematocrit values should be followed to rule out bleeding from the ulcer.
Adverse Reactions
Clinical Trials Experience
The following-adverse reactions have been observed, but there is not enough data to support an estimate of frequency.
Cardiovascular: Tachycardia, palpitation.
Allergic: Severe allergic reaction or drug idiosyncrasies including anaphylaxis.
CNS: Headaches, nervousness, mental confusion, drowsiness, dizziness.
Special Senses: Blurred vision, dilation of the pupil, cycloplegia, increased ocular tension, loss of taste.
Renal: Urinary hesitancy and retention.
Gastrointestinal: Nausea, vomiting, constipation, bloated feeling.
Dermatologic: Decreased sweating, urticaria and other dermal manifestations.
Miscellaneous: Xerostomia, weakness, insomnia, impotence, suppression of lactation.
Postmarketing Experience
There is limited information regarding Methscopolamine bromide Postmarketing Experience in the drug label.
Drug Interactions
Additive anticholinergic effects may result from concomitant use with antipsychotics, tricyclic antidepressants, and other drugs with anticholinergic effects. Concomitant administration with antacids may interfere with the absorption of methscopolamine bromide.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with methscopolamine bromide. It also is not known whether methscopolamine bromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methscopolamine bromide should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methscopolamine bromide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methscopolamine bromide during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methscopolamine bromide is administered to a nursing woman.
Pediatric Use
Safety and efficacy in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Methscopolamine bromide in geriatric settings.
Gender
There is no FDA guidance on the use of Methscopolamine bromide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methscopolamine bromide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methscopolamine bromide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methscopolamine bromide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methscopolamine bromide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methscopolamine bromide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Methscopolamine bromide Administration in the drug label.
Monitoring
There is limited information regarding Methscopolamine bromide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Methscopolamine bromide and IV administrations.
Overdosage
The symptoms of overdosage with Methscopolamine Bromide 2.5 mg Tablets/Methscopolamine Bromide 5 mg Tablets progress from intensification of the usual side effects to CNS disturbances (from restlessness and excitement to psychotic behavior), circulatory changes (flushing, fall in blood pressure, circulatory failure), respiratory failure, paralysis, and coma.
Measures to be taken are (1) induction of emesis and (2) injection of physostigmine 0.5 to 2 mg intravenously, and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (alcohol sponging, ice packs). Excitement of a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 ml of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
The oral LD50 in rats is 1,352 to 2,617 mg/kg.
No data is available on the dialyzability of methscopolamine bromide.
Pharmacology
| |
Methscopolamine bromide
| |
| Systematic (IUPAC) name | |
| (1R,2S,4R,5S,7R)-{[(2R)-3-hydroxy-2-phenylpropanoyl]oxy}-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane | |
| Identifiers | |
| CAS number | |
| ATC code | A03 S01FA03 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 318.388 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 3–4 hrs |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Methscopolamine bromide Mechanism of Action in the drug label.
Structure
There is limited information regarding Methscopolamine bromide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Methscopolamine bromide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Methscopolamine bromide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Methscopolamine bromide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Methscopolamine bromide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Methscopolamine bromide How Supplied in the drug label.
Storage
There is limited information regarding Methscopolamine bromide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Methscopolamine bromide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Methscopolamine bromide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Methscopolamine bromide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Methscopolamine bromide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Methscopolamine bromide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Methscopolamine bromide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.