Ingenol mebutate: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= {{VP}} <!--Overview--> |genericName= |aOrAn= an |drugClass= inducer of cell death |indication= actinic keratosis |hasBl...") |
No edit summary |
||
| Line 45: | Line 45: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Actinic Keratosis===== | ||
* | *For topical use only; Picato® gel is not for oral, ophthalmic, or intravaginal use. | ||
*For the treatment of actinic keratosis on the face and scalp Picato® gel, 0.015% should be applied to the affected area once daily for 3 consecutive days. | |||
* | *For the treatment of actinic keratosis on the trunk and extremities Picato® gel, 0.05% should be applied to the affected area once daily for 2 consecutive days. | ||
*Picato® gel may be applied to the affected area, up to one contiguous skin area of approximately 25 cm2 (e.g., 5 cm x 5 cm) using one unit dose tube. After spreading evenly over the treatment area, the gel should be allowed to dry for 15 minutes. Patients should wash their hands immediately after applying Picato® gel and take care not to transfer the applied drug to other areas, including the eye. Patients should avoid washing and touching the treated area for a period of 6 hours after application of Picato® gel. Following this time, patients may wash the area with a mild soap. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 161: | Line 147: | ||
|contraindications= | |contraindications= | ||
* | * None. | ||
<!--Warnings--> | <!--Warnings--> | ||
| Line 167: | Line 153: | ||
|warnings= | |warnings= | ||
====Precautions==== | |||
* Eye Exposure | |||
:*Eye disorders, including severe eye pain, eyelid edema, eyelid ptosis, periorbital edema can occur after exposure. Patients should wash hands well after applying Picato® gel, and avoid transfer of the drug to the periocular area during and after application. If accidental exposure occurs, the area should be flushed with water and the patient should seek medical care as soon as possible. | |||
* | *Local Skin Reactions | ||
:*Severe skin reactions in the treated area, including erythema, crusting, swelling, vesiculation/postulation, and erosion/ulceration, can occur after topical application of Picato® gel. Administration of Picato® gel is not recommended until the skin is healed from any previous drug or surgical treatment. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 179: | Line 167: | ||
|clinicalTrials= | |clinicalTrials= | ||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
*The data described below reflect exposure to Picato® gel in 499 subjects with actinic keratosis, including 274 subjects exposed to Picato® gel field treatment (skin area of 25 cm2 in the face or scalp regions) at a concentration of 0.015% once daily for 3 consecutive days, and 225 subjects exposed to Picato® gel field treatment (skin area of 25 cm2 in the trunk or extremities regions) at a concentration of 0.05% once daily for 2 consecutive days. | |||
*Local skin reactions, including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration were assessed within the selected treatment area and graded by the investigator on a scale of 0 to 4. A grade of 0 represented no reaction present in the treated area, and a grade of 4 indicated a marked and severe skin reaction that extended beyond the treated area. | |||
T1 | |||
T2 | |||
*Local skin reactions typically occurred within 1 day of treatment initiation, peaked in intensity up to 1 week following completion of treatment, and resolved within 2 weeks for areas treated on the face and scalp, and within 4 weeks for areas treated on the trunk and extremities. | |||
*Adverse reactions that occurred in ≥2% of subjects treated with Picato® gel and at a higher frequency than the vehicle are presented in Table 3 and Table 4. | |||
T3 | |||
T4 | |||
*Less common adverse reactions in subjects treated with Picato® included: eyelid edema, eye pain, conjunctivitis. | |||
*A total of 108 subjects treated with Picato® gel on the face/scalp and 38 subjects treated on the trunk/extremities were followed for 12 months. Results from these studies did not change the safety profile of Picato® gel. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 250: | Line 194: | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions= | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category C''' | ||
*There are no adequate and well-controlled studies of Picato® gel in pregnant women. Picato® gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
*Systemic embryofetal development studies were conducted with ingenol mebutate in rats and rabbits. Intravenous doses of 1.5, 3, and 5 µg/kg/day (9, 18, and 30 μg/m2/day) ingenol mebutate were administered during the period of organogenesis (gestational days 6 – 16) to pregnant female rats. No treatment related effects on embryofetal toxicity or teratogenicity were noted at doses up to 5 µg/kg/day (30 μg/m2/day). Intravenous doses of 1, 2, and 4 µg/kg/day (12, 24, and 48 μg/m2/day) ingenol mebutate were administered during the period of organogenesis (gestational days 6 – 18) to pregnant female rabbits. An increase in embryo-fetal mortality was noted at 4 µg/kg/day (48 μg/m2/day). An increased incidence of fetal visceral and skeletal variations was noted in all three ingenol mebutate dose groups. The clinical relevance of these findings is unclear since systemic exposure of ingenol mebutate was not detected in subjects with actinic keratosis treated with Picato® gel, 0.05% applied to a 100 cm2 treatment area [see Clinical Pharmacology ( 12.3)]. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 327: | Line 220: | ||
|useInPed= | |useInPed= | ||
*Actinic keratosis is not a condition generally seen within the pediatric population. | |||
*The safety and effectiveness of Picato® gel for actinic keratosis in patients less than 18 years of age have not been established. | |||
|useInGeri= | |useInGeri= | ||
*Of the 1165 subjects treated with Picato® gel in the clinical trials, 56% were 65 years and older and, 21% were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. | |||
|useInGender= | |useInGender= | ||
| Line 354: | Line 251: | ||
|administration= | |administration= | ||
* | * Topical | ||
|monitoring= | |monitoring= | ||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 376: | Line 269: | ||
===Acute Overdose=== | ===Acute Overdose=== | ||
*Topical overdosing of Picato® gel could result in an increased incidence of local skin reactions. | |||
* | |||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 394: | Line 281: | ||
|drugBox= | |drugBox= | ||
{{Drugbox2 | |||
| IUPAC_name = (1a''R'',2''S'',5''R'',5a''S'',6S,8a''S'',9''R'',10a''R'')-5,5a-Dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1''H''-2,8a-methanocyclopenta[''a'']cyclpropa[''e''][10]annulen-6-yl (2''Z'')-2-methylbut-2-enoate | |||
| image = Ingenol mebutate.png | |||
| alt = | |||
| caption = | |||
<!-- Clinical data --> | |||
| tradename = Picato | |||
| Drugs.com = {{Drugs.com|parent|picato}} | |||
| MedlinePlus = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = C | |||
| pregnancy_category= | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM --> | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = Topical (gel) | |||
<!-- Pharmacokinetic data --> | |||
| bioavailability = Below detection level | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!-- Identifiers --> | |||
| CAS_number = 75567-37-2 | |||
| ATCvet = | |||
| ATC_prefix = D06 | |||
| ATC_suffix = BX02 | |||
| PubChem = 6918670 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 66913 | |||
| DrugBank = | |||
| ChemSpiderID = 26325194 | |||
| synonyms = PEP005, ingenol-3-angelate | |||
<!-- Chemical data --> | |||
| C=25 | H=34 | O=6 | |||
| molecular_weight = 430.534 g/mol | |||
| StdInChI=1S/C25H34O6/c1-7-12(2)22(29)31-21-13(3)10-24-14(4)8-17-18(23(17,5)6)16(20(24)28)9-15(11-26)19(27)25(21,24)30/h7,9-10,14,16-19,21,26-27,30H,8,11H2,1-6H3/b12-7-/t14-,16+,17-,18+,19-,21+,24+,25+/m1/s1 | |||
| StdInChIKey = VDJHFHXMUKFKET-WDUFCVPESA-N | |||
| smiles = C/C=C(/C)\C(=O)O[C@H]1C(=C[C@@]23[C@@]1([C@@H](C(=C[C@H](C2=O)[C@H]4[C@H](C4(C)C)C[C@H]3C)CO)O)O)C | |||
}} | |||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
| Line 400: | Line 332: | ||
|mechAction= | |mechAction= | ||
* | * The mechanism of action by which Picato® gel induces cell death in treating AK lesions is unknown. | ||
<!--Structure--> | <!--Structure--> | ||
| Line 406: | Line 338: | ||
|structure= | |structure= | ||
* | * Picato® (ingenol mebutate) gel, 0.015% or 0.05% is a clear colorless gel for topical administration, which contains the active substance ingenol mebutate, an inducer of cell death. | ||
*The chemical name of ingenol mebutate is: | |||
:*2-Butenoic acid, 2-methyl-, (1aR,2S,5R,5aS,6S,8aS,9R,10aR)-1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1H-2,8a-methanocyclopenta [a]cyclopropa[e]cyclodecen-6-yl ester, (2Z) - | |||
or | |||
:*(1aR,2S,5R,5aS,6S,8aS,9R,10aR)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1H 2,8a-methanocyclopenta[a]cyclopropa[e]cyclodecen-6-yl (2Z) 2 methylbut-2-enoate. | |||
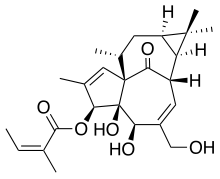
*The molecular formula is C25H34O6 and molecular weight is 430.5. Ingenol mebutate is represented by the following structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Ingenol mebutate is a white to pale yellow crystalline powder. | |||
*Picato® gel, 0.015% and 0.05% contains 150 mcg and 500 mcg of ingenol mebutate, respectively in each gram of gel consisting of isopropyl alcohol, hydroxyethyl cellulose, citric acid monohydrate, sodium citrate, benzyl alcohol and purified water. | |||
*Picato® gel is clear colorless gel and supplied in unit dose laminate tubes, for single use, containing a nominal fill weight of 0.47 g, with a deliverable weight of 0.25 g. The tubes should be discarded after single use. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
Revision as of 14:37, 13 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ingenol mebutate is an inducer of cell death that is FDA approved for the {{{indicationType}}} of actinic keratosis. Common adverse reactions include local skin reactions, application site pain, application site pruritus, application site irritation, application site infection, periorbital edema, nasopharyngitis and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Actinic Keratosis
- For topical use only; Picato® gel is not for oral, ophthalmic, or intravaginal use.
- For the treatment of actinic keratosis on the face and scalp Picato® gel, 0.015% should be applied to the affected area once daily for 3 consecutive days.
- For the treatment of actinic keratosis on the trunk and extremities Picato® gel, 0.05% should be applied to the affected area once daily for 2 consecutive days.
- Picato® gel may be applied to the affected area, up to one contiguous skin area of approximately 25 cm2 (e.g., 5 cm x 5 cm) using one unit dose tube. After spreading evenly over the treatment area, the gel should be allowed to dry for 15 minutes. Patients should wash their hands immediately after applying Picato® gel and take care not to transfer the applied drug to other areas, including the eye. Patients should avoid washing and touching the treated area for a period of 6 hours after application of Picato® gel. Following this time, patients may wash the area with a mild soap.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ingenol mebutate in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ingenol mebutate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Ingenol mebutate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ingenol mebutate in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ingenol mebutate in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- Eye Exposure
- Eye disorders, including severe eye pain, eyelid edema, eyelid ptosis, periorbital edema can occur after exposure. Patients should wash hands well after applying Picato® gel, and avoid transfer of the drug to the periocular area during and after application. If accidental exposure occurs, the area should be flushed with water and the patient should seek medical care as soon as possible.
- Local Skin Reactions
- Severe skin reactions in the treated area, including erythema, crusting, swelling, vesiculation/postulation, and erosion/ulceration, can occur after topical application of Picato® gel. Administration of Picato® gel is not recommended until the skin is healed from any previous drug or surgical treatment.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The data described below reflect exposure to Picato® gel in 499 subjects with actinic keratosis, including 274 subjects exposed to Picato® gel field treatment (skin area of 25 cm2 in the face or scalp regions) at a concentration of 0.015% once daily for 3 consecutive days, and 225 subjects exposed to Picato® gel field treatment (skin area of 25 cm2 in the trunk or extremities regions) at a concentration of 0.05% once daily for 2 consecutive days.
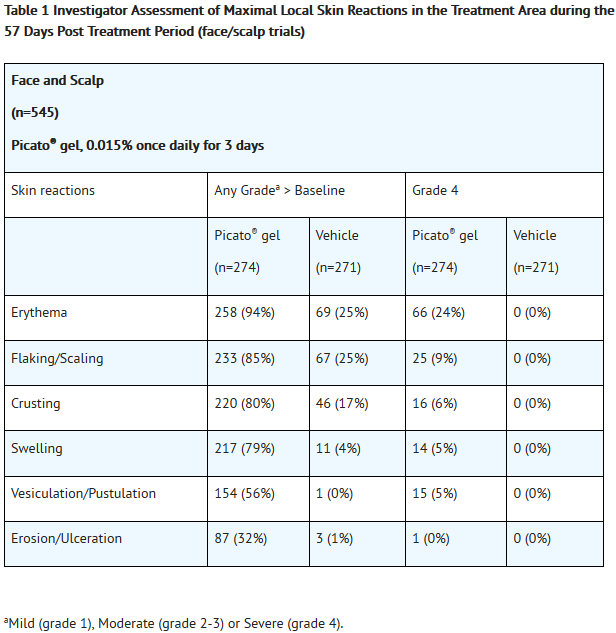
- Local skin reactions, including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration were assessed within the selected treatment area and graded by the investigator on a scale of 0 to 4. A grade of 0 represented no reaction present in the treated area, and a grade of 4 indicated a marked and severe skin reaction that extended beyond the treated area.
T1
T2
- Local skin reactions typically occurred within 1 day of treatment initiation, peaked in intensity up to 1 week following completion of treatment, and resolved within 2 weeks for areas treated on the face and scalp, and within 4 weeks for areas treated on the trunk and extremities.
- Adverse reactions that occurred in ≥2% of subjects treated with Picato® gel and at a higher frequency than the vehicle are presented in Table 3 and Table 4.
T3
T4
- Less common adverse reactions in subjects treated with Picato® included: eyelid edema, eye pain, conjunctivitis.
- A total of 108 subjects treated with Picato® gel on the face/scalp and 38 subjects treated on the trunk/extremities were followed for 12 months. Results from these studies did not change the safety profile of Picato® gel.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ingenol mebutate in the drug label.
Drug Interactions
There is limited information regarding Ingenol mebutate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well-controlled studies of Picato® gel in pregnant women. Picato® gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Systemic embryofetal development studies were conducted with ingenol mebutate in rats and rabbits. Intravenous doses of 1.5, 3, and 5 µg/kg/day (9, 18, and 30 μg/m2/day) ingenol mebutate were administered during the period of organogenesis (gestational days 6 – 16) to pregnant female rats. No treatment related effects on embryofetal toxicity or teratogenicity were noted at doses up to 5 µg/kg/day (30 μg/m2/day). Intravenous doses of 1, 2, and 4 µg/kg/day (12, 24, and 48 μg/m2/day) ingenol mebutate were administered during the period of organogenesis (gestational days 6 – 18) to pregnant female rabbits. An increase in embryo-fetal mortality was noted at 4 µg/kg/day (48 μg/m2/day). An increased incidence of fetal visceral and skeletal variations was noted in all three ingenol mebutate dose groups. The clinical relevance of these findings is unclear since systemic exposure of ingenol mebutate was not detected in subjects with actinic keratosis treated with Picato® gel, 0.05% applied to a 100 cm2 treatment area [see Clinical Pharmacology ( 12.3)].
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ingenol mebutate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ingenol mebutate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ingenol mebutate with respect to nursing mothers.
Pediatric Use
- Actinic keratosis is not a condition generally seen within the pediatric population.
- The safety and effectiveness of Picato® gel for actinic keratosis in patients less than 18 years of age have not been established.
Geriatic Use
- Of the 1165 subjects treated with Picato® gel in the clinical trials, 56% were 65 years and older and, 21% were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Gender
There is no FDA guidance on the use of Ingenol mebutate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ingenol mebutate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ingenol mebutate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ingenol mebutate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ingenol mebutate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ingenol mebutate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Ingenol mebutate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ingenol mebutate in the drug label.
Overdosage
Acute Overdose
- Topical overdosing of Picato® gel could result in an increased incidence of local skin reactions.
Chronic Overdose
There is limited information regarding Chronic Overdose of Ingenol mebutate in the drug label.
Pharmacology
Mechanism of Action
- The mechanism of action by which Picato® gel induces cell death in treating AK lesions is unknown.
Structure
- Picato® (ingenol mebutate) gel, 0.015% or 0.05% is a clear colorless gel for topical administration, which contains the active substance ingenol mebutate, an inducer of cell death.
- The chemical name of ingenol mebutate is:
- 2-Butenoic acid, 2-methyl-, (1aR,2S,5R,5aS,6S,8aS,9R,10aR)-1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1H-2,8a-methanocyclopenta [a]cyclopropa[e]cyclodecen-6-yl ester, (2Z) -
or
- (1aR,2S,5R,5aS,6S,8aS,9R,10aR)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1H 2,8a-methanocyclopenta[a]cyclopropa[e]cyclodecen-6-yl (2Z) 2 methylbut-2-enoate.
- The molecular formula is C25H34O6 and molecular weight is 430.5. Ingenol mebutate is represented by the following structural formula:
- Ingenol mebutate is a white to pale yellow crystalline powder.
- Picato® gel, 0.015% and 0.05% contains 150 mcg and 500 mcg of ingenol mebutate, respectively in each gram of gel consisting of isopropyl alcohol, hydroxyethyl cellulose, citric acid monohydrate, sodium citrate, benzyl alcohol and purified water.
- Picato® gel is clear colorless gel and supplied in unit dose laminate tubes, for single use, containing a nominal fill weight of 0.47 g, with a deliverable weight of 0.25 g. The tubes should be discarded after single use.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ingenol mebutate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ingenol mebutate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ingenol mebutate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ingenol mebutate in the drug label.
How Supplied
Storage
There is limited information regarding Ingenol mebutate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ingenol mebutate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ingenol mebutate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Ingenol mebutate in the drug label.
Precautions with Alcohol
- Alcohol-Ingenol mebutate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ingenol mebutate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Ingenol mebutate |Label Name=Ingenol mebutate11.png
}}
{{#subobject:
|Label Page=Ingenol mebutate |Label Name=Ingenol mebutate11.png
}}